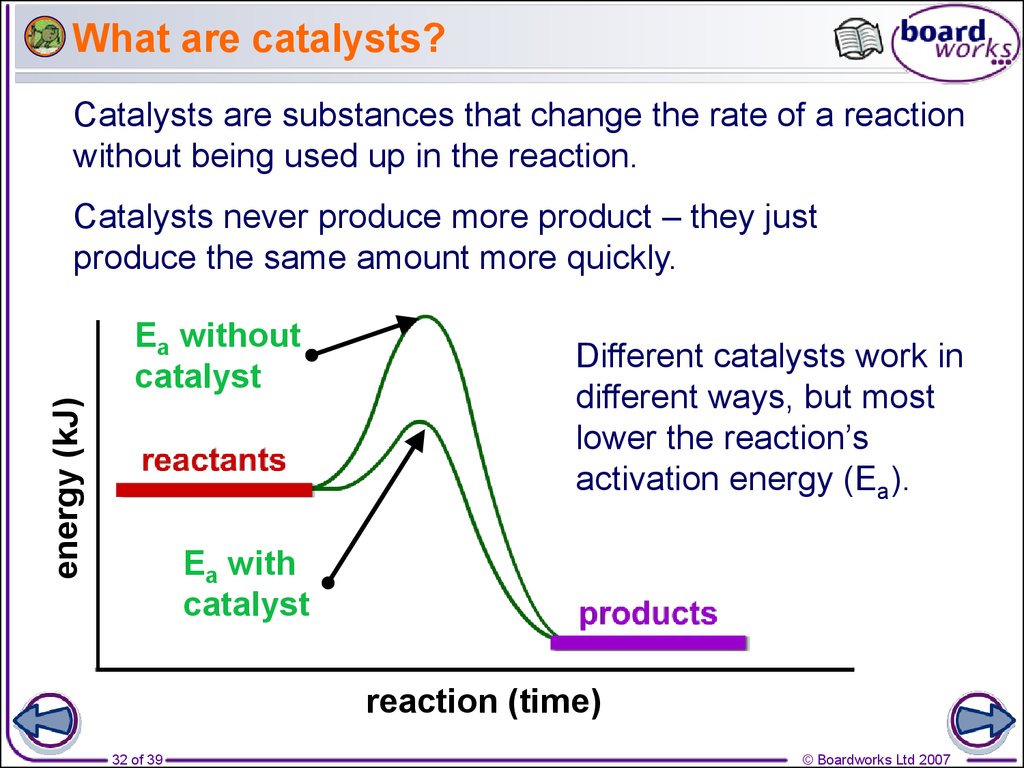
Do Catalysts Affect Rate Law . Catalysts, which do not appear in the balanced overall chemical equation, can also influence reaction rate. It assumes that you are already familiar with basic ideas about the collision theory of reaction. The rate of a chemical reaction is determined—and altered—by many factors, including the nature (of reactivity) of reactants, surface area, temperature, concentration, and catalysts. Catalysis describes the process by which the rate of a reaction is increased by the addition of a substance known as a catalyst. Catalysts are substances that speed up a reaction but which are not consumed by it and do not appear in the net reaction equation. This page describes and explains the way that adding a catalyst affects the rate of a reaction. A catalysed reaction produces the same amount of product. The rate law is experimentally. This page explains how adding a catalyst affects the rate of a reaction. It assumes familiarity with basic concepts in the collision theory of reaction rates, and with the maxwell. It is possible for a catalyst to change the order of a reaction since it shows different path to the reaction i.e.
from ppt-online.org
A catalysed reaction produces the same amount of product. This page explains how adding a catalyst affects the rate of a reaction. Catalysis describes the process by which the rate of a reaction is increased by the addition of a substance known as a catalyst. The rate of a chemical reaction is determined—and altered—by many factors, including the nature (of reactivity) of reactants, surface area, temperature, concentration, and catalysts. Catalysts are substances that speed up a reaction but which are not consumed by it and do not appear in the net reaction equation. It is possible for a catalyst to change the order of a reaction since it shows different path to the reaction i.e. Catalysts, which do not appear in the balanced overall chemical equation, can also influence reaction rate. It assumes familiarity with basic concepts in the collision theory of reaction rates, and with the maxwell. It assumes that you are already familiar with basic ideas about the collision theory of reaction. The rate law is experimentally.
Rates of reaction презентация онлайн
Do Catalysts Affect Rate Law Catalysts, which do not appear in the balanced overall chemical equation, can also influence reaction rate. It assumes familiarity with basic concepts in the collision theory of reaction rates, and with the maxwell. This page explains how adding a catalyst affects the rate of a reaction. Catalysis describes the process by which the rate of a reaction is increased by the addition of a substance known as a catalyst. It assumes that you are already familiar with basic ideas about the collision theory of reaction. This page describes and explains the way that adding a catalyst affects the rate of a reaction. A catalysed reaction produces the same amount of product. The rate of a chemical reaction is determined—and altered—by many factors, including the nature (of reactivity) of reactants, surface area, temperature, concentration, and catalysts. The rate law is experimentally. Catalysts are substances that speed up a reaction but which are not consumed by it and do not appear in the net reaction equation. Catalysts, which do not appear in the balanced overall chemical equation, can also influence reaction rate. It is possible for a catalyst to change the order of a reaction since it shows different path to the reaction i.e.
From www.youtube.com
Writing Rate Laws of Reaction Mechanisms Using The Rate Determining Do Catalysts Affect Rate Law Catalysts, which do not appear in the balanced overall chemical equation, can also influence reaction rate. Catalysts are substances that speed up a reaction but which are not consumed by it and do not appear in the net reaction equation. A catalysed reaction produces the same amount of product. The rate of a chemical reaction is determined—and altered—by many factors,. Do Catalysts Affect Rate Law.
From www.youtube.com
Catalyst Affects Reaction Rate Energy Diagram with a Catalyst YouTube Do Catalysts Affect Rate Law Catalysts are substances that speed up a reaction but which are not consumed by it and do not appear in the net reaction equation. The rate law is experimentally. A catalysed reaction produces the same amount of product. Catalysis describes the process by which the rate of a reaction is increased by the addition of a substance known as a. Do Catalysts Affect Rate Law.
From www.slideserve.com
PPT Factors That Affect the Rate of a Reaction PowerPoint Do Catalysts Affect Rate Law The rate law is experimentally. Catalysts, which do not appear in the balanced overall chemical equation, can also influence reaction rate. It is possible for a catalyst to change the order of a reaction since it shows different path to the reaction i.e. This page explains how adding a catalyst affects the rate of a reaction. Catalysts are substances that. Do Catalysts Affect Rate Law.
From www.slideshare.net
8.1 rate law Do Catalysts Affect Rate Law This page explains how adding a catalyst affects the rate of a reaction. The rate law is experimentally. It is possible for a catalyst to change the order of a reaction since it shows different path to the reaction i.e. Catalysts, which do not appear in the balanced overall chemical equation, can also influence reaction rate. Catalysts are substances that. Do Catalysts Affect Rate Law.
From edu.rsc.org
How do catalysts affect reaction rates? 1618 years Lesson plan Do Catalysts Affect Rate Law It assumes familiarity with basic concepts in the collision theory of reaction rates, and with the maxwell. The rate law is experimentally. It assumes that you are already familiar with basic ideas about the collision theory of reaction. Catalysts are substances that speed up a reaction but which are not consumed by it and do not appear in the net. Do Catalysts Affect Rate Law.
From www.slideserve.com
PPT Reaction Rates PowerPoint Presentation, free download ID6050384 Do Catalysts Affect Rate Law Catalysts, which do not appear in the balanced overall chemical equation, can also influence reaction rate. The rate of a chemical reaction is determined—and altered—by many factors, including the nature (of reactivity) of reactants, surface area, temperature, concentration, and catalysts. It assumes familiarity with basic concepts in the collision theory of reaction rates, and with the maxwell. Catalysis describes the. Do Catalysts Affect Rate Law.
From www.youtube.com
Rate Laws and the Method of Initial Rates YouTube Do Catalysts Affect Rate Law Catalysts are substances that speed up a reaction but which are not consumed by it and do not appear in the net reaction equation. Catalysts, which do not appear in the balanced overall chemical equation, can also influence reaction rate. It assumes that you are already familiar with basic ideas about the collision theory of reaction. The rate of a. Do Catalysts Affect Rate Law.
From www.waca.msf.org
Lesson Explainer Catalysts, Catalyst Do Catalysts Affect Rate Law This page describes and explains the way that adding a catalyst affects the rate of a reaction. The rate law is experimentally. Catalysts, which do not appear in the balanced overall chemical equation, can also influence reaction rate. This page explains how adding a catalyst affects the rate of a reaction. A catalysed reaction produces the same amount of product.. Do Catalysts Affect Rate Law.
From www.slideserve.com
PPT CATALYSIS AND CATALYTIC REACTION MECHANISM PART 1 PowerPoint Do Catalysts Affect Rate Law It assumes familiarity with basic concepts in the collision theory of reaction rates, and with the maxwell. This page describes and explains the way that adding a catalyst affects the rate of a reaction. It assumes that you are already familiar with basic ideas about the collision theory of reaction. The rate of a chemical reaction is determined—and altered—by many. Do Catalysts Affect Rate Law.
From www.slideshare.net
1.4 rate of reaction(1.2d)...biology Do Catalysts Affect Rate Law It is possible for a catalyst to change the order of a reaction since it shows different path to the reaction i.e. This page describes and explains the way that adding a catalyst affects the rate of a reaction. A catalysed reaction produces the same amount of product. Catalysis describes the process by which the rate of a reaction is. Do Catalysts Affect Rate Law.
From www.meritnation.com
Illustrate graphically the effect of a catalyst on rate of a reaction Do Catalysts Affect Rate Law Catalysts are substances that speed up a reaction but which are not consumed by it and do not appear in the net reaction equation. Catalysis describes the process by which the rate of a reaction is increased by the addition of a substance known as a catalyst. This page describes and explains the way that adding a catalyst affects the. Do Catalysts Affect Rate Law.
From slideplayer.com
Chemical How are chemical reaction rates determined and what Do Catalysts Affect Rate Law This page describes and explains the way that adding a catalyst affects the rate of a reaction. Catalysis describes the process by which the rate of a reaction is increased by the addition of a substance known as a catalyst. A catalysed reaction produces the same amount of product. Catalysts, which do not appear in the balanced overall chemical equation,. Do Catalysts Affect Rate Law.
From slideplayer.com
Chapter 13 Chemical ppt download Do Catalysts Affect Rate Law Catalysts are substances that speed up a reaction but which are not consumed by it and do not appear in the net reaction equation. Catalysts, which do not appear in the balanced overall chemical equation, can also influence reaction rate. It is possible for a catalyst to change the order of a reaction since it shows different path to the. Do Catalysts Affect Rate Law.
From www.slideserve.com
PPT RATES OF REACTION A guide for GCSE students PowerPoint Do Catalysts Affect Rate Law Catalysis describes the process by which the rate of a reaction is increased by the addition of a substance known as a catalyst. Catalysts, which do not appear in the balanced overall chemical equation, can also influence reaction rate. The rate law is experimentally. It is possible for a catalyst to change the order of a reaction since it shows. Do Catalysts Affect Rate Law.
From slideplayer.com
Factors Affecting the Rate of Reaction ppt download Do Catalysts Affect Rate Law Catalysts are substances that speed up a reaction but which are not consumed by it and do not appear in the net reaction equation. It assumes that you are already familiar with basic ideas about the collision theory of reaction. Catalysis describes the process by which the rate of a reaction is increased by the addition of a substance known. Do Catalysts Affect Rate Law.
From www.youtube.com
How Catalysts Affect Rate Of Reaction GCSE Chemistry Do Catalysts Affect Rate Law This page explains how adding a catalyst affects the rate of a reaction. The rate law is experimentally. Catalysts, which do not appear in the balanced overall chemical equation, can also influence reaction rate. Catalysis describes the process by which the rate of a reaction is increased by the addition of a substance known as a catalyst. It is possible. Do Catalysts Affect Rate Law.
From www.nagwa.com
Question Video Describing the Effect of Catalysts on Reaction Rates Do Catalysts Affect Rate Law A catalysed reaction produces the same amount of product. This page explains how adding a catalyst affects the rate of a reaction. The rate of a chemical reaction is determined—and altered—by many factors, including the nature (of reactivity) of reactants, surface area, temperature, concentration, and catalysts. It is possible for a catalyst to change the order of a reaction since. Do Catalysts Affect Rate Law.
From wiringfixunripping.z21.web.core.windows.net
Reaction Energy Diagram With Catalyst Do Catalysts Affect Rate Law This page explains how adding a catalyst affects the rate of a reaction. Catalysts, which do not appear in the balanced overall chemical equation, can also influence reaction rate. The rate of a chemical reaction is determined—and altered—by many factors, including the nature (of reactivity) of reactants, surface area, temperature, concentration, and catalysts. It assumes familiarity with basic concepts in. Do Catalysts Affect Rate Law.
From www.slideserve.com
PPT Rates of Reactions PowerPoint Presentation, free download ID Do Catalysts Affect Rate Law This page describes and explains the way that adding a catalyst affects the rate of a reaction. Catalysts are substances that speed up a reaction but which are not consumed by it and do not appear in the net reaction equation. Catalysis describes the process by which the rate of a reaction is increased by the addition of a substance. Do Catalysts Affect Rate Law.
From jackwestin.com
Rate Processes Catalysts Rate Processes In Chemical Reactions Do Catalysts Affect Rate Law Catalysts are substances that speed up a reaction but which are not consumed by it and do not appear in the net reaction equation. Catalysts, which do not appear in the balanced overall chemical equation, can also influence reaction rate. A catalysed reaction produces the same amount of product. It assumes that you are already familiar with basic ideas about. Do Catalysts Affect Rate Law.
From www.slideserve.com
PPT Reaction mechanisms and catalysts PowerPoint Presentation, free Do Catalysts Affect Rate Law The rate law is experimentally. This page describes and explains the way that adding a catalyst affects the rate of a reaction. It assumes that you are already familiar with basic ideas about the collision theory of reaction. This page explains how adding a catalyst affects the rate of a reaction. Catalysts, which do not appear in the balanced overall. Do Catalysts Affect Rate Law.
From slideplayer.com
Chapter 2 The Chemistry of Life. ppt download Do Catalysts Affect Rate Law Catalysts, which do not appear in the balanced overall chemical equation, can also influence reaction rate. This page describes and explains the way that adding a catalyst affects the rate of a reaction. This page explains how adding a catalyst affects the rate of a reaction. It assumes that you are already familiar with basic ideas about the collision theory. Do Catalysts Affect Rate Law.
From slideplayer.com
Lecture 1401 Factors that Affect Reaction Rates ppt download Do Catalysts Affect Rate Law It is possible for a catalyst to change the order of a reaction since it shows different path to the reaction i.e. This page explains how adding a catalyst affects the rate of a reaction. The rate law is experimentally. It assumes that you are already familiar with basic ideas about the collision theory of reaction. A catalysed reaction produces. Do Catalysts Affect Rate Law.
From slideplayer.com
Unit 5 Chapter ppt download Do Catalysts Affect Rate Law A catalysed reaction produces the same amount of product. This page describes and explains the way that adding a catalyst affects the rate of a reaction. This page explains how adding a catalyst affects the rate of a reaction. The rate of a chemical reaction is determined—and altered—by many factors, including the nature (of reactivity) of reactants, surface area, temperature,. Do Catalysts Affect Rate Law.
From www.mometrix.com
What is a Catalyst? Chemistry Review (Video) Do Catalysts Affect Rate Law A catalysed reaction produces the same amount of product. It is possible for a catalyst to change the order of a reaction since it shows different path to the reaction i.e. The rate law is experimentally. Catalysts are substances that speed up a reaction but which are not consumed by it and do not appear in the net reaction equation.. Do Catalysts Affect Rate Law.
From byjus.com
How does a catalyst increase the rate of a reaction? Do Catalysts Affect Rate Law It assumes familiarity with basic concepts in the collision theory of reaction rates, and with the maxwell. Catalysis describes the process by which the rate of a reaction is increased by the addition of a substance known as a catalyst. This page describes and explains the way that adding a catalyst affects the rate of a reaction. Catalysts, which do. Do Catalysts Affect Rate Law.
From sciencenotes.org
What Is a Catalyst? Understand Catalysis Do Catalysts Affect Rate Law This page explains how adding a catalyst affects the rate of a reaction. Catalysts are substances that speed up a reaction but which are not consumed by it and do not appear in the net reaction equation. The rate law is experimentally. The rate of a chemical reaction is determined—and altered—by many factors, including the nature (of reactivity) of reactants,. Do Catalysts Affect Rate Law.
From www.youtube.com
Factors Affecting the Rate of Reaction Catalyst Rate of Reaction Do Catalysts Affect Rate Law Catalysis describes the process by which the rate of a reaction is increased by the addition of a substance known as a catalyst. The rate law is experimentally. Catalysts, which do not appear in the balanced overall chemical equation, can also influence reaction rate. A catalysed reaction produces the same amount of product. It assumes familiarity with basic concepts in. Do Catalysts Affect Rate Law.
From www.youtube.com
How to find the rate law graphically and how to use the integrated rate Do Catalysts Affect Rate Law It assumes familiarity with basic concepts in the collision theory of reaction rates, and with the maxwell. This page explains how adding a catalyst affects the rate of a reaction. This page describes and explains the way that adding a catalyst affects the rate of a reaction. Catalysts are substances that speed up a reaction but which are not consumed. Do Catalysts Affect Rate Law.
From byjus.com
Why do catalyst not affect equilibrium? Do Catalysts Affect Rate Law It is possible for a catalyst to change the order of a reaction since it shows different path to the reaction i.e. The rate of a chemical reaction is determined—and altered—by many factors, including the nature (of reactivity) of reactants, surface area, temperature, concentration, and catalysts. Catalysts are substances that speed up a reaction but which are not consumed by. Do Catalysts Affect Rate Law.
From www.slideserve.com
PPT Rate of Reaction PowerPoint Presentation, free download ID2483456 Do Catalysts Affect Rate Law Catalysis describes the process by which the rate of a reaction is increased by the addition of a substance known as a catalyst. It assumes familiarity with basic concepts in the collision theory of reaction rates, and with the maxwell. This page describes and explains the way that adding a catalyst affects the rate of a reaction. The rate of. Do Catalysts Affect Rate Law.
From www.slideserve.com
PPT Reaction mechanisms and catalysts PowerPoint Presentation, free Do Catalysts Affect Rate Law Catalysis describes the process by which the rate of a reaction is increased by the addition of a substance known as a catalyst. This page explains how adding a catalyst affects the rate of a reaction. A catalysed reaction produces the same amount of product. The rate of a chemical reaction is determined—and altered—by many factors, including the nature (of. Do Catalysts Affect Rate Law.
From www.nagwa.com
Question Video Understanding How Catalysts Affect the Rates and the Do Catalysts Affect Rate Law Catalysts, which do not appear in the balanced overall chemical equation, can also influence reaction rate. A catalysed reaction produces the same amount of product. It assumes that you are already familiar with basic ideas about the collision theory of reaction. Catalysts are substances that speed up a reaction but which are not consumed by it and do not appear. Do Catalysts Affect Rate Law.
From slideplayer.com
Unit 5 Chemical ppt download Do Catalysts Affect Rate Law The rate of a chemical reaction is determined—and altered—by many factors, including the nature (of reactivity) of reactants, surface area, temperature, concentration, and catalysts. It is possible for a catalyst to change the order of a reaction since it shows different path to the reaction i.e. A catalysed reaction produces the same amount of product. This page describes and explains. Do Catalysts Affect Rate Law.
From ppt-online.org
Rates of reaction презентация онлайн Do Catalysts Affect Rate Law Catalysts are substances that speed up a reaction but which are not consumed by it and do not appear in the net reaction equation. It assumes familiarity with basic concepts in the collision theory of reaction rates, and with the maxwell. Catalysts, which do not appear in the balanced overall chemical equation, can also influence reaction rate. It is possible. Do Catalysts Affect Rate Law.