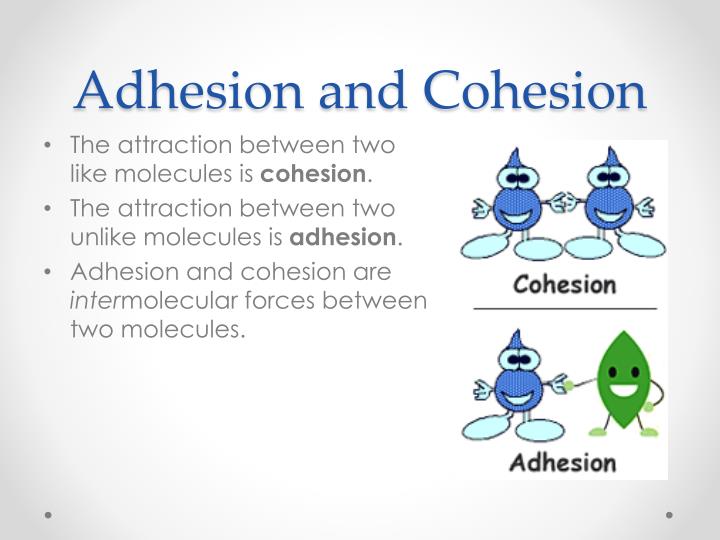
Surface Tension Of Water Cohesion . cohesion allows for the development of surface tension, the capacity of a substance to withstand being ruptured when placed under tension or stress. As the bristles dry out, the surface tension effect dissipates. the surface tension of the water surrounding the bristles is sufficient to hold the bristles together. The cohesive forces between molecules in a liquid are shared with all neighboring molecules. cohesive forces between molecules cause the surface of a liquid to contract to the smallest possible surface area. surface tension in water might be good at performing tricks, such as being able to float a paper clip on its surface, but surface tension performs. This general effect is called. cohesion and surface tension.
from www.slideserve.com
cohesion allows for the development of surface tension, the capacity of a substance to withstand being ruptured when placed under tension or stress. This general effect is called. cohesive forces between molecules cause the surface of a liquid to contract to the smallest possible surface area. As the bristles dry out, the surface tension effect dissipates. surface tension in water might be good at performing tricks, such as being able to float a paper clip on its surface, but surface tension performs. the surface tension of the water surrounding the bristles is sufficient to hold the bristles together. cohesion and surface tension. The cohesive forces between molecules in a liquid are shared with all neighboring molecules.
PPT Lesson 3 Cohesion, adhesion & surface tension PowerPoint
Surface Tension Of Water Cohesion cohesion allows for the development of surface tension, the capacity of a substance to withstand being ruptured when placed under tension or stress. cohesion and surface tension. the surface tension of the water surrounding the bristles is sufficient to hold the bristles together. cohesive forces between molecules cause the surface of a liquid to contract to the smallest possible surface area. cohesion allows for the development of surface tension, the capacity of a substance to withstand being ruptured when placed under tension or stress. This general effect is called. As the bristles dry out, the surface tension effect dissipates. The cohesive forces between molecules in a liquid are shared with all neighboring molecules. surface tension in water might be good at performing tricks, such as being able to float a paper clip on its surface, but surface tension performs.
From studiousguy.com
10 Surface Tension Examples in Daily Life StudiousGuy Surface Tension Of Water Cohesion As the bristles dry out, the surface tension effect dissipates. cohesive forces between molecules cause the surface of a liquid to contract to the smallest possible surface area. the surface tension of the water surrounding the bristles is sufficient to hold the bristles together. The cohesive forces between molecules in a liquid are shared with all neighboring molecules.. Surface Tension Of Water Cohesion.
From aniya-has-pitts.blogspot.com
Surface Tension and Cohesion Occur in Pure Water Because Water Aniya Surface Tension Of Water Cohesion As the bristles dry out, the surface tension effect dissipates. surface tension in water might be good at performing tricks, such as being able to float a paper clip on its surface, but surface tension performs. cohesive forces between molecules cause the surface of a liquid to contract to the smallest possible surface area. cohesion allows for. Surface Tension Of Water Cohesion.
From www.slideserve.com
PPT Water PowerPoint Presentation, free download ID2600281 Surface Tension Of Water Cohesion surface tension in water might be good at performing tricks, such as being able to float a paper clip on its surface, but surface tension performs. The cohesive forces between molecules in a liquid are shared with all neighboring molecules. the surface tension of the water surrounding the bristles is sufficient to hold the bristles together. As the. Surface Tension Of Water Cohesion.
From www.thoughtco.com
Surface Tension Definition in Chemistry Surface Tension Of Water Cohesion The cohesive forces between molecules in a liquid are shared with all neighboring molecules. surface tension in water might be good at performing tricks, such as being able to float a paper clip on its surface, but surface tension performs. cohesive forces between molecules cause the surface of a liquid to contract to the smallest possible surface area.. Surface Tension Of Water Cohesion.
From www.animalia-life.club
Cohesion Of Water Diagram Surface Tension Of Water Cohesion surface tension in water might be good at performing tricks, such as being able to float a paper clip on its surface, but surface tension performs. cohesion and surface tension. cohesion allows for the development of surface tension, the capacity of a substance to withstand being ruptured when placed under tension or stress. the surface tension. Surface Tension Of Water Cohesion.
From www.haikudeck.com
Biology by Illiana Leppert Surface Tension Of Water Cohesion As the bristles dry out, the surface tension effect dissipates. cohesive forces between molecules cause the surface of a liquid to contract to the smallest possible surface area. the surface tension of the water surrounding the bristles is sufficient to hold the bristles together. cohesion and surface tension. surface tension in water might be good at. Surface Tension Of Water Cohesion.
From www.slideserve.com
PPT Properties of Water PowerPoint Presentation, free download ID Surface Tension Of Water Cohesion the surface tension of the water surrounding the bristles is sufficient to hold the bristles together. surface tension in water might be good at performing tricks, such as being able to float a paper clip on its surface, but surface tension performs. cohesion and surface tension. As the bristles dry out, the surface tension effect dissipates. The. Surface Tension Of Water Cohesion.
From www.slideserve.com
PPT Water Cohesion and Surface Tension PowerPoint Presentation, free Surface Tension Of Water Cohesion surface tension in water might be good at performing tricks, such as being able to float a paper clip on its surface, but surface tension performs. cohesion allows for the development of surface tension, the capacity of a substance to withstand being ruptured when placed under tension or stress. cohesive forces between molecules cause the surface of. Surface Tension Of Water Cohesion.
From www.animalia-life.club
Cohesion Of Water Diagram Surface Tension Of Water Cohesion cohesion allows for the development of surface tension, the capacity of a substance to withstand being ruptured when placed under tension or stress. As the bristles dry out, the surface tension effect dissipates. cohesive forces between molecules cause the surface of a liquid to contract to the smallest possible surface area. The cohesive forces between molecules in a. Surface Tension Of Water Cohesion.
From chem.libretexts.org
Chapter 7.3 Unique Properties of Liquids Chemistry LibreTexts Surface Tension Of Water Cohesion As the bristles dry out, the surface tension effect dissipates. the surface tension of the water surrounding the bristles is sufficient to hold the bristles together. cohesion and surface tension. cohesion allows for the development of surface tension, the capacity of a substance to withstand being ruptured when placed under tension or stress. This general effect is. Surface Tension Of Water Cohesion.
From mungfali.com
What Is Cohesion In Water Surface Tension Of Water Cohesion This general effect is called. surface tension in water might be good at performing tricks, such as being able to float a paper clip on its surface, but surface tension performs. As the bristles dry out, the surface tension effect dissipates. cohesive forces between molecules cause the surface of a liquid to contract to the smallest possible surface. Surface Tension Of Water Cohesion.
From www.biolinscientific.com
Surface tension of water Why is it so high? Surface Tension Of Water Cohesion The cohesive forces between molecules in a liquid are shared with all neighboring molecules. As the bristles dry out, the surface tension effect dissipates. cohesion allows for the development of surface tension, the capacity of a substance to withstand being ruptured when placed under tension or stress. the surface tension of the water surrounding the bristles is sufficient. Surface Tension Of Water Cohesion.
From www.youtube.com
Cohesion, Adhesion, & Surface Tension YouTube Surface Tension Of Water Cohesion surface tension in water might be good at performing tricks, such as being able to float a paper clip on its surface, but surface tension performs. cohesion and surface tension. This general effect is called. The cohesive forces between molecules in a liquid are shared with all neighboring molecules. cohesive forces between molecules cause the surface of. Surface Tension Of Water Cohesion.
From www.slideserve.com
PPT Unit 4 The Hydrosphere PowerPoint Presentation, free download Surface Tension Of Water Cohesion surface tension in water might be good at performing tricks, such as being able to float a paper clip on its surface, but surface tension performs. cohesion and surface tension. cohesive forces between molecules cause the surface of a liquid to contract to the smallest possible surface area. cohesion allows for the development of surface tension,. Surface Tension Of Water Cohesion.
From www.slideserve.com
PPT Chapter 3 Water and Life PowerPoint Presentation, free download Surface Tension Of Water Cohesion the surface tension of the water surrounding the bristles is sufficient to hold the bristles together. surface tension in water might be good at performing tricks, such as being able to float a paper clip on its surface, but surface tension performs. cohesion and surface tension. cohesion allows for the development of surface tension, the capacity. Surface Tension Of Water Cohesion.
From bio.libretexts.org
2.16 Water Cohesive and Adhesive Properties Biology LibreTexts Surface Tension Of Water Cohesion As the bristles dry out, the surface tension effect dissipates. cohesive forces between molecules cause the surface of a liquid to contract to the smallest possible surface area. surface tension in water might be good at performing tricks, such as being able to float a paper clip on its surface, but surface tension performs. The cohesive forces between. Surface Tension Of Water Cohesion.
From jason-kirwin.blogspot.com
Describe the Cohesiontension Theory of Water Transport in the Xylem Surface Tension Of Water Cohesion As the bristles dry out, the surface tension effect dissipates. The cohesive forces between molecules in a liquid are shared with all neighboring molecules. cohesion and surface tension. cohesive forces between molecules cause the surface of a liquid to contract to the smallest possible surface area. cohesion allows for the development of surface tension, the capacity of. Surface Tension Of Water Cohesion.
From www.thoughtco.com
Cohesion Definition and Examples in Chemistry Surface Tension Of Water Cohesion cohesion allows for the development of surface tension, the capacity of a substance to withstand being ruptured when placed under tension or stress. surface tension in water might be good at performing tricks, such as being able to float a paper clip on its surface, but surface tension performs. The cohesive forces between molecules in a liquid are. Surface Tension Of Water Cohesion.
From www.expii.com
Cohesion and Adhesion (Water) — Properties & Examples Expii Surface Tension Of Water Cohesion cohesion allows for the development of surface tension, the capacity of a substance to withstand being ruptured when placed under tension or stress. As the bristles dry out, the surface tension effect dissipates. This general effect is called. surface tension in water might be good at performing tricks, such as being able to float a paper clip on. Surface Tension Of Water Cohesion.
From www.slideserve.com
PPT Water (H2O) PowerPoint Presentation, free download ID1700557 Surface Tension Of Water Cohesion As the bristles dry out, the surface tension effect dissipates. cohesion and surface tension. The cohesive forces between molecules in a liquid are shared with all neighboring molecules. cohesive forces between molecules cause the surface of a liquid to contract to the smallest possible surface area. the surface tension of the water surrounding the bristles is sufficient. Surface Tension Of Water Cohesion.
From www.slideshare.net
Measuring the Surface Tension of Water by Light Diffraction on Capill… Surface Tension Of Water Cohesion surface tension in water might be good at performing tricks, such as being able to float a paper clip on its surface, but surface tension performs. The cohesive forces between molecules in a liquid are shared with all neighboring molecules. the surface tension of the water surrounding the bristles is sufficient to hold the bristles together. cohesive. Surface Tension Of Water Cohesion.
From ar.inspiredpencil.com
The Relationship Between Hydrogen Bonding And Surface Tension, Adhesion Surface Tension Of Water Cohesion surface tension in water might be good at performing tricks, such as being able to float a paper clip on its surface, but surface tension performs. This general effect is called. cohesion allows for the development of surface tension, the capacity of a substance to withstand being ruptured when placed under tension or stress. The cohesive forces between. Surface Tension Of Water Cohesion.
From www.slideserve.com
PPT Water (H2O) PowerPoint Presentation, free download ID1700557 Surface Tension Of Water Cohesion The cohesive forces between molecules in a liquid are shared with all neighboring molecules. cohesive forces between molecules cause the surface of a liquid to contract to the smallest possible surface area. This general effect is called. cohesion allows for the development of surface tension, the capacity of a substance to withstand being ruptured when placed under tension. Surface Tension Of Water Cohesion.
From www.slideserve.com
PPT Cohesion and Surface Tension PowerPoint Presentation, free Surface Tension Of Water Cohesion This general effect is called. surface tension in water might be good at performing tricks, such as being able to float a paper clip on its surface, but surface tension performs. cohesive forces between molecules cause the surface of a liquid to contract to the smallest possible surface area. the surface tension of the water surrounding the. Surface Tension Of Water Cohesion.
From www.slideserve.com
PPT Cohesion and Surface Tension PowerPoint Presentation, free Surface Tension Of Water Cohesion surface tension in water might be good at performing tricks, such as being able to float a paper clip on its surface, but surface tension performs. This general effect is called. cohesive forces between molecules cause the surface of a liquid to contract to the smallest possible surface area. The cohesive forces between molecules in a liquid are. Surface Tension Of Water Cohesion.
From www.animalia-life.club
Water Cohesion Diagram Surface Tension Of Water Cohesion cohesive forces between molecules cause the surface of a liquid to contract to the smallest possible surface area. cohesion and surface tension. As the bristles dry out, the surface tension effect dissipates. surface tension in water might be good at performing tricks, such as being able to float a paper clip on its surface, but surface tension. Surface Tension Of Water Cohesion.
From www.slideserve.com
PPT Lesson 3 Cohesion, adhesion & surface tension PowerPoint Surface Tension Of Water Cohesion As the bristles dry out, the surface tension effect dissipates. surface tension in water might be good at performing tricks, such as being able to float a paper clip on its surface, but surface tension performs. the surface tension of the water surrounding the bristles is sufficient to hold the bristles together. cohesive forces between molecules cause. Surface Tension Of Water Cohesion.
From www.slideserve.com
PPT Unique Properties of water PowerPoint Presentation, free download Surface Tension Of Water Cohesion The cohesive forces between molecules in a liquid are shared with all neighboring molecules. As the bristles dry out, the surface tension effect dissipates. cohesive forces between molecules cause the surface of a liquid to contract to the smallest possible surface area. surface tension in water might be good at performing tricks, such as being able to float. Surface Tension Of Water Cohesion.
From stock.adobe.com
Example of Surface tension. Water cohesive forces of the surface layer Surface Tension Of Water Cohesion The cohesive forces between molecules in a liquid are shared with all neighboring molecules. cohesion and surface tension. cohesion allows for the development of surface tension, the capacity of a substance to withstand being ruptured when placed under tension or stress. This general effect is called. cohesive forces between molecules cause the surface of a liquid to. Surface Tension Of Water Cohesion.
From stock.adobe.com
illustration of physics, Surface tension of water, the cohesive forces Surface Tension Of Water Cohesion As the bristles dry out, the surface tension effect dissipates. The cohesive forces between molecules in a liquid are shared with all neighboring molecules. surface tension in water might be good at performing tricks, such as being able to float a paper clip on its surface, but surface tension performs. This general effect is called. cohesion and surface. Surface Tension Of Water Cohesion.
From www.aakash.ac.in
Surface Tension Definition, Causes, Measurement & Formula AESL Surface Tension Of Water Cohesion the surface tension of the water surrounding the bristles is sufficient to hold the bristles together. As the bristles dry out, the surface tension effect dissipates. This general effect is called. cohesion and surface tension. cohesion allows for the development of surface tension, the capacity of a substance to withstand being ruptured when placed under tension or. Surface Tension Of Water Cohesion.
From www.slideserve.com
PPT Lesson 3 Cohesion, adhesion & surface tension PowerPoint Surface Tension Of Water Cohesion As the bristles dry out, the surface tension effect dissipates. the surface tension of the water surrounding the bristles is sufficient to hold the bristles together. cohesion allows for the development of surface tension, the capacity of a substance to withstand being ruptured when placed under tension or stress. cohesion and surface tension. The cohesive forces between. Surface Tension Of Water Cohesion.
From www.animalia-life.club
Water Cohesion Diagram Surface Tension Of Water Cohesion the surface tension of the water surrounding the bristles is sufficient to hold the bristles together. The cohesive forces between molecules in a liquid are shared with all neighboring molecules. cohesive forces between molecules cause the surface of a liquid to contract to the smallest possible surface area. As the bristles dry out, the surface tension effect dissipates.. Surface Tension Of Water Cohesion.
From bio.libretexts.org
4.5.1.3 CohesionTension Theory Biology LibreTexts Surface Tension Of Water Cohesion cohesive forces between molecules cause the surface of a liquid to contract to the smallest possible surface area. cohesion and surface tension. surface tension in water might be good at performing tricks, such as being able to float a paper clip on its surface, but surface tension performs. cohesion allows for the development of surface tension,. Surface Tension Of Water Cohesion.
From www.scienceabc.com
Surface Tension Definition, Explanation, Examples And Significance Surface Tension Of Water Cohesion As the bristles dry out, the surface tension effect dissipates. cohesive forces between molecules cause the surface of a liquid to contract to the smallest possible surface area. The cohesive forces between molecules in a liquid are shared with all neighboring molecules. cohesion and surface tension. the surface tension of the water surrounding the bristles is sufficient. Surface Tension Of Water Cohesion.