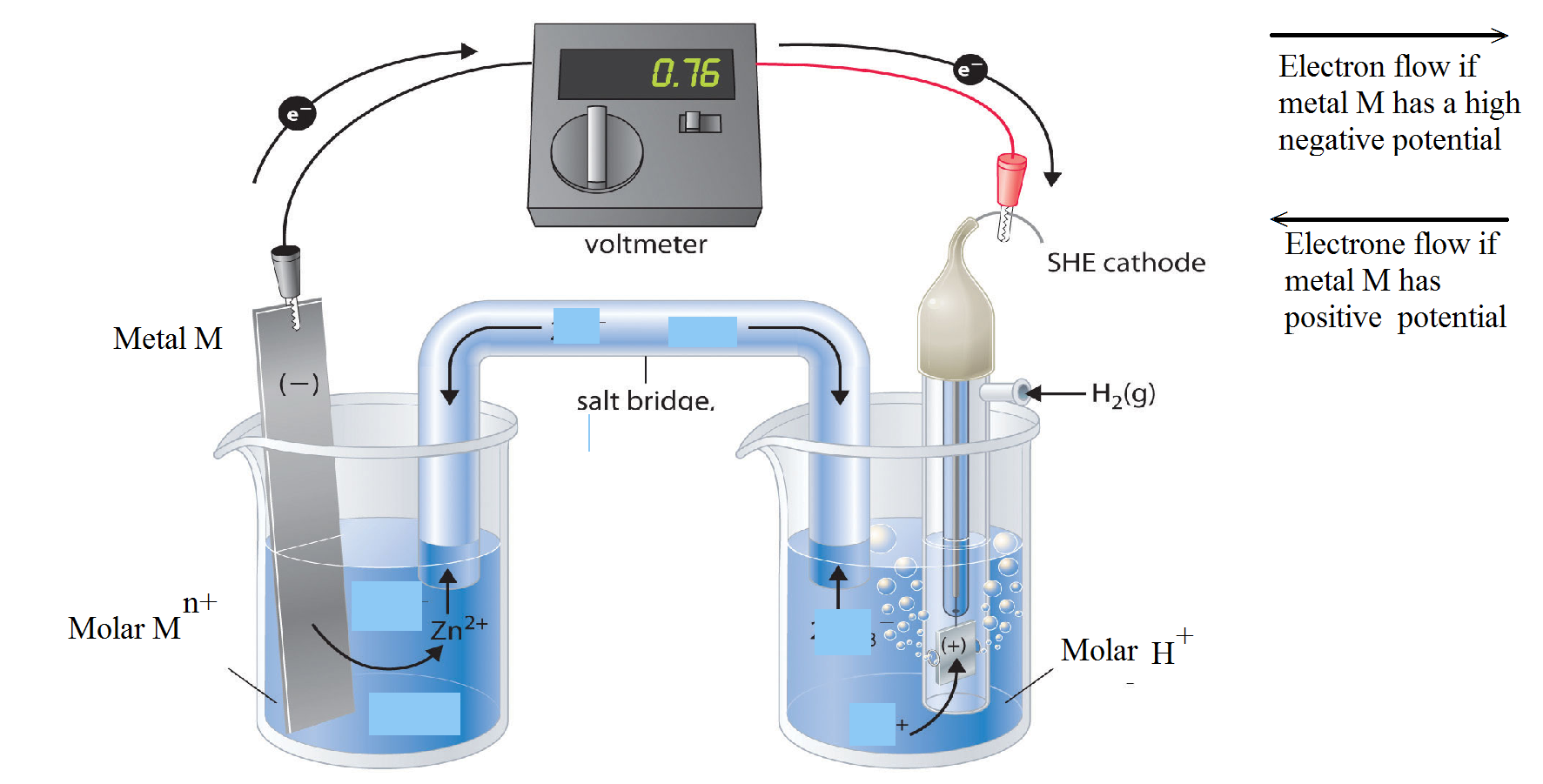
Hydrogen Electrode Concentration Cell . The structure of the standard hydrogen electrode is. An introduction to redox equilibria and electrode potentials. This page explains the background to standard electrode potentials (redox potentials), showing how they. An example for this type of cell would be a cell consisting of two hydrogen electrodes which are subjected to varying pressures but are immersed in the same solutions (containing hydrogen. A concentration cell is an electrochemical cell where both electrodes are made of the same material, but they are immersed in electrolytes. An electrochemical cell of this type, in which the anode and cathode compartments are identical except for the concentration of a reactant, is called a concentration cell.
from www.askiitians.com
This page explains the background to standard electrode potentials (redox potentials), showing how they. An introduction to redox equilibria and electrode potentials. A concentration cell is an electrochemical cell where both electrodes are made of the same material, but they are immersed in electrolytes. An example for this type of cell would be a cell consisting of two hydrogen electrodes which are subjected to varying pressures but are immersed in the same solutions (containing hydrogen. An electrochemical cell of this type, in which the anode and cathode compartments are identical except for the concentration of a reactant, is called a concentration cell. The structure of the standard hydrogen electrode is.
Electrode Potential Study Material for IIT JEE askIITians
Hydrogen Electrode Concentration Cell A concentration cell is an electrochemical cell where both electrodes are made of the same material, but they are immersed in electrolytes. The structure of the standard hydrogen electrode is. A concentration cell is an electrochemical cell where both electrodes are made of the same material, but they are immersed in electrolytes. An example for this type of cell would be a cell consisting of two hydrogen electrodes which are subjected to varying pressures but are immersed in the same solutions (containing hydrogen. An electrochemical cell of this type, in which the anode and cathode compartments are identical except for the concentration of a reactant, is called a concentration cell. An introduction to redox equilibria and electrode potentials. This page explains the background to standard electrode potentials (redox potentials), showing how they.
From www.slideserve.com
PPT Lecture 13 The Nernst Equation PowerPoint Presentation, free Hydrogen Electrode Concentration Cell A concentration cell is an electrochemical cell where both electrodes are made of the same material, but they are immersed in electrolytes. An example for this type of cell would be a cell consisting of two hydrogen electrodes which are subjected to varying pressures but are immersed in the same solutions (containing hydrogen. An introduction to redox equilibria and electrode. Hydrogen Electrode Concentration Cell.
From www.researchgate.net
Figure The anode and cathode reactions in typical electrolytic Hydrogen Electrode Concentration Cell A concentration cell is an electrochemical cell where both electrodes are made of the same material, but they are immersed in electrolytes. This page explains the background to standard electrode potentials (redox potentials), showing how they. An introduction to redox equilibria and electrode potentials. An electrochemical cell of this type, in which the anode and cathode compartments are identical except. Hydrogen Electrode Concentration Cell.
From www.chegg.com
Solved HW28.1. Concentration Cells Pt electrode atoms Ht Hydrogen Electrode Concentration Cell An example for this type of cell would be a cell consisting of two hydrogen electrodes which are subjected to varying pressures but are immersed in the same solutions (containing hydrogen. This page explains the background to standard electrode potentials (redox potentials), showing how they. The structure of the standard hydrogen electrode is. An electrochemical cell of this type, in. Hydrogen Electrode Concentration Cell.
From www.youtube.com
Lec30 Electrode Concentration Cells EMF Derivation Nernst Equation Hydrogen Electrode Concentration Cell A concentration cell is an electrochemical cell where both electrodes are made of the same material, but they are immersed in electrolytes. The structure of the standard hydrogen electrode is. This page explains the background to standard electrode potentials (redox potentials), showing how they. An example for this type of cell would be a cell consisting of two hydrogen electrodes. Hydrogen Electrode Concentration Cell.
From www.slideserve.com
PPT CHEM1612 Pharmacy Week 9 Concentration Cells PowerPoint Hydrogen Electrode Concentration Cell This page explains the background to standard electrode potentials (redox potentials), showing how they. A concentration cell is an electrochemical cell where both electrodes are made of the same material, but they are immersed in electrolytes. The structure of the standard hydrogen electrode is. An electrochemical cell of this type, in which the anode and cathode compartments are identical except. Hydrogen Electrode Concentration Cell.
From byjus.com
Standard Hydrogen Electrode Definition, Construction, and Labelled Hydrogen Electrode Concentration Cell This page explains the background to standard electrode potentials (redox potentials), showing how they. A concentration cell is an electrochemical cell where both electrodes are made of the same material, but they are immersed in electrolytes. An introduction to redox equilibria and electrode potentials. An electrochemical cell of this type, in which the anode and cathode compartments are identical except. Hydrogen Electrode Concentration Cell.
From saylordotorg.github.io
Electrochemistry Hydrogen Electrode Concentration Cell This page explains the background to standard electrode potentials (redox potentials), showing how they. An example for this type of cell would be a cell consisting of two hydrogen electrodes which are subjected to varying pressures but are immersed in the same solutions (containing hydrogen. The structure of the standard hydrogen electrode is. An electrochemical cell of this type, in. Hydrogen Electrode Concentration Cell.
From www.numerade.com
SOLVEDWhat is the cell potential of a concentration cell that contains Hydrogen Electrode Concentration Cell This page explains the background to standard electrode potentials (redox potentials), showing how they. An example for this type of cell would be a cell consisting of two hydrogen electrodes which are subjected to varying pressures but are immersed in the same solutions (containing hydrogen. The structure of the standard hydrogen electrode is. An introduction to redox equilibria and electrode. Hydrogen Electrode Concentration Cell.
From www.numerade.com
SOLVEDA concentration cell is constructed of two hydrogen electrodes Hydrogen Electrode Concentration Cell A concentration cell is an electrochemical cell where both electrodes are made of the same material, but they are immersed in electrolytes. An example for this type of cell would be a cell consisting of two hydrogen electrodes which are subjected to varying pressures but are immersed in the same solutions (containing hydrogen. The structure of the standard hydrogen electrode. Hydrogen Electrode Concentration Cell.
From www.oceangeothermal.org
Hydrogen Through Electrolysis Ocean Geothermal Energy Foundation Hydrogen Electrode Concentration Cell An example for this type of cell would be a cell consisting of two hydrogen electrodes which are subjected to varying pressures but are immersed in the same solutions (containing hydrogen. The structure of the standard hydrogen electrode is. An electrochemical cell of this type, in which the anode and cathode compartments are identical except for the concentration of a. Hydrogen Electrode Concentration Cell.
From www.chemicals.co.uk
A Level Chemistry Electrodes & Electrochemical Cells Hydrogen Electrode Concentration Cell An electrochemical cell of this type, in which the anode and cathode compartments are identical except for the concentration of a reactant, is called a concentration cell. A concentration cell is an electrochemical cell where both electrodes are made of the same material, but they are immersed in electrolytes. This page explains the background to standard electrode potentials (redox potentials),. Hydrogen Electrode Concentration Cell.
From www.youtube.com
Standard hydrogen electrode ( SHE ) , EC CELL, NERNST EQUATION BY Hydrogen Electrode Concentration Cell This page explains the background to standard electrode potentials (redox potentials), showing how they. A concentration cell is an electrochemical cell where both electrodes are made of the same material, but they are immersed in electrolytes. An electrochemical cell of this type, in which the anode and cathode compartments are identical except for the concentration of a reactant, is called. Hydrogen Electrode Concentration Cell.
From saylordotorg.github.io
Electrochemistry Hydrogen Electrode Concentration Cell The structure of the standard hydrogen electrode is. A concentration cell is an electrochemical cell where both electrodes are made of the same material, but they are immersed in electrolytes. An electrochemical cell of this type, in which the anode and cathode compartments are identical except for the concentration of a reactant, is called a concentration cell. This page explains. Hydrogen Electrode Concentration Cell.
From monomole.com
Standard hydrogen electrode Mono Mole Hydrogen Electrode Concentration Cell An introduction to redox equilibria and electrode potentials. This page explains the background to standard electrode potentials (redox potentials), showing how they. The structure of the standard hydrogen electrode is. An example for this type of cell would be a cell consisting of two hydrogen electrodes which are subjected to varying pressures but are immersed in the same solutions (containing. Hydrogen Electrode Concentration Cell.
From www.echemi.com
What is the physical explanation driving current flow in a Hydrogen Electrode Concentration Cell An example for this type of cell would be a cell consisting of two hydrogen electrodes which are subjected to varying pressures but are immersed in the same solutions (containing hydrogen. The structure of the standard hydrogen electrode is. A concentration cell is an electrochemical cell where both electrodes are made of the same material, but they are immersed in. Hydrogen Electrode Concentration Cell.
From www.expii.com
Concentration Cells — Overview & Applications Expii Hydrogen Electrode Concentration Cell A concentration cell is an electrochemical cell where both electrodes are made of the same material, but they are immersed in electrolytes. This page explains the background to standard electrode potentials (redox potentials), showing how they. An electrochemical cell of this type, in which the anode and cathode compartments are identical except for the concentration of a reactant, is called. Hydrogen Electrode Concentration Cell.
From www.youtube.com
Electrochemistry 10. How to find potential of hydrogen electrode Hydrogen Electrode Concentration Cell A concentration cell is an electrochemical cell where both electrodes are made of the same material, but they are immersed in electrolytes. An introduction to redox equilibria and electrode potentials. This page explains the background to standard electrode potentials (redox potentials), showing how they. An electrochemical cell of this type, in which the anode and cathode compartments are identical except. Hydrogen Electrode Concentration Cell.
From www.numerade.com
SOLVED A concentration cell consisting of two hydrogen electrodes (P H Hydrogen Electrode Concentration Cell An electrochemical cell of this type, in which the anode and cathode compartments are identical except for the concentration of a reactant, is called a concentration cell. The structure of the standard hydrogen electrode is. An example for this type of cell would be a cell consisting of two hydrogen electrodes which are subjected to varying pressures but are immersed. Hydrogen Electrode Concentration Cell.
From www.youtube.com
C.6 Concentration cells (HL) YouTube Hydrogen Electrode Concentration Cell This page explains the background to standard electrode potentials (redox potentials), showing how they. The structure of the standard hydrogen electrode is. A concentration cell is an electrochemical cell where both electrodes are made of the same material, but they are immersed in electrolytes. An introduction to redox equilibria and electrode potentials. An example for this type of cell would. Hydrogen Electrode Concentration Cell.
From greenenergymaterial.com
Basics Of Electrochemical Cells » Green Energy Material Hydrogen Electrode Concentration Cell A concentration cell is an electrochemical cell where both electrodes are made of the same material, but they are immersed in electrolytes. The structure of the standard hydrogen electrode is. An electrochemical cell of this type, in which the anode and cathode compartments are identical except for the concentration of a reactant, is called a concentration cell. An example for. Hydrogen Electrode Concentration Cell.
From gaskatel.de
Principles of the pHvalue measurement with hydrogen electrodes Gaskatel Hydrogen Electrode Concentration Cell This page explains the background to standard electrode potentials (redox potentials), showing how they. An example for this type of cell would be a cell consisting of two hydrogen electrodes which are subjected to varying pressures but are immersed in the same solutions (containing hydrogen. The structure of the standard hydrogen electrode is. An introduction to redox equilibria and electrode. Hydrogen Electrode Concentration Cell.
From saylordotorg.github.io
Electrochemistry Hydrogen Electrode Concentration Cell An electrochemical cell of this type, in which the anode and cathode compartments are identical except for the concentration of a reactant, is called a concentration cell. The structure of the standard hydrogen electrode is. A concentration cell is an electrochemical cell where both electrodes are made of the same material, but they are immersed in electrolytes. An introduction to. Hydrogen Electrode Concentration Cell.
From stock.adobe.com
Standard hydrogen electrode diagram. Scientific vector illustration Hydrogen Electrode Concentration Cell An electrochemical cell of this type, in which the anode and cathode compartments are identical except for the concentration of a reactant, is called a concentration cell. This page explains the background to standard electrode potentials (redox potentials), showing how they. An introduction to redox equilibria and electrode potentials. A concentration cell is an electrochemical cell where both electrodes are. Hydrogen Electrode Concentration Cell.
From saylordotorg.github.io
Electrochemistry Hydrogen Electrode Concentration Cell A concentration cell is an electrochemical cell where both electrodes are made of the same material, but they are immersed in electrolytes. An electrochemical cell of this type, in which the anode and cathode compartments are identical except for the concentration of a reactant, is called a concentration cell. An example for this type of cell would be a cell. Hydrogen Electrode Concentration Cell.
From app.pandai.org
Standard Electrode Potential Hydrogen Electrode Concentration Cell This page explains the background to standard electrode potentials (redox potentials), showing how they. The structure of the standard hydrogen electrode is. An example for this type of cell would be a cell consisting of two hydrogen electrodes which are subjected to varying pressures but are immersed in the same solutions (containing hydrogen. An electrochemical cell of this type, in. Hydrogen Electrode Concentration Cell.
From www.researchgate.net
Schematic of a high temperature hydrogen electrode concentration cell Hydrogen Electrode Concentration Cell A concentration cell is an electrochemical cell where both electrodes are made of the same material, but they are immersed in electrolytes. An introduction to redox equilibria and electrode potentials. An electrochemical cell of this type, in which the anode and cathode compartments are identical except for the concentration of a reactant, is called a concentration cell. The structure of. Hydrogen Electrode Concentration Cell.
From www.youtube.com
Electrochemistry The Concentration Cell. YouTube Hydrogen Electrode Concentration Cell This page explains the background to standard electrode potentials (redox potentials), showing how they. An electrochemical cell of this type, in which the anode and cathode compartments are identical except for the concentration of a reactant, is called a concentration cell. An example for this type of cell would be a cell consisting of two hydrogen electrodes which are subjected. Hydrogen Electrode Concentration Cell.
From www.youtube.com
EMF of Electrode Concentration Cell Reversible to Cations (cell with Hydrogen Electrode Concentration Cell This page explains the background to standard electrode potentials (redox potentials), showing how they. The structure of the standard hydrogen electrode is. An introduction to redox equilibria and electrode potentials. An electrochemical cell of this type, in which the anode and cathode compartments are identical except for the concentration of a reactant, is called a concentration cell. An example for. Hydrogen Electrode Concentration Cell.
From chem.libretexts.org
Chapter 19.1 Describing Electrochemical Cells Chemistry LibreTexts Hydrogen Electrode Concentration Cell A concentration cell is an electrochemical cell where both electrodes are made of the same material, but they are immersed in electrolytes. An introduction to redox equilibria and electrode potentials. The structure of the standard hydrogen electrode is. An example for this type of cell would be a cell consisting of two hydrogen electrodes which are subjected to varying pressures. Hydrogen Electrode Concentration Cell.
From pubs.acs.org
Standard and Reversible Hydrogen Electrodes Theory, Design, Operation Hydrogen Electrode Concentration Cell This page explains the background to standard electrode potentials (redox potentials), showing how they. An electrochemical cell of this type, in which the anode and cathode compartments are identical except for the concentration of a reactant, is called a concentration cell. An example for this type of cell would be a cell consisting of two hydrogen electrodes which are subjected. Hydrogen Electrode Concentration Cell.
From question.pandai.org
Standard Electrode Potential Hydrogen Electrode Concentration Cell The structure of the standard hydrogen electrode is. A concentration cell is an electrochemical cell where both electrodes are made of the same material, but they are immersed in electrolytes. An example for this type of cell would be a cell consisting of two hydrogen electrodes which are subjected to varying pressures but are immersed in the same solutions (containing. Hydrogen Electrode Concentration Cell.
From www.slideserve.com
PPT Chemistry 142 Chapter 18 Electrochemistry PowerPoint Hydrogen Electrode Concentration Cell A concentration cell is an electrochemical cell where both electrodes are made of the same material, but they are immersed in electrolytes. This page explains the background to standard electrode potentials (redox potentials), showing how they. The structure of the standard hydrogen electrode is. An electrochemical cell of this type, in which the anode and cathode compartments are identical except. Hydrogen Electrode Concentration Cell.
From askfilo.com
Electrode concentration cell Emf develops between two IIKe dipped in th.. Hydrogen Electrode Concentration Cell This page explains the background to standard electrode potentials (redox potentials), showing how they. The structure of the standard hydrogen electrode is. An example for this type of cell would be a cell consisting of two hydrogen electrodes which are subjected to varying pressures but are immersed in the same solutions (containing hydrogen. An introduction to redox equilibria and electrode. Hydrogen Electrode Concentration Cell.
From www.askiitians.com
Electrode Potential Study Material for IIT JEE askIITians Hydrogen Electrode Concentration Cell The structure of the standard hydrogen electrode is. An example for this type of cell would be a cell consisting of two hydrogen electrodes which are subjected to varying pressures but are immersed in the same solutions (containing hydrogen. A concentration cell is an electrochemical cell where both electrodes are made of the same material, but they are immersed in. Hydrogen Electrode Concentration Cell.
From courses.lumenlearning.com
17.3 Standard Reduction Potentials General College Chemistry II Hydrogen Electrode Concentration Cell This page explains the background to standard electrode potentials (redox potentials), showing how they. The structure of the standard hydrogen electrode is. An electrochemical cell of this type, in which the anode and cathode compartments are identical except for the concentration of a reactant, is called a concentration cell. An introduction to redox equilibria and electrode potentials. A concentration cell. Hydrogen Electrode Concentration Cell.