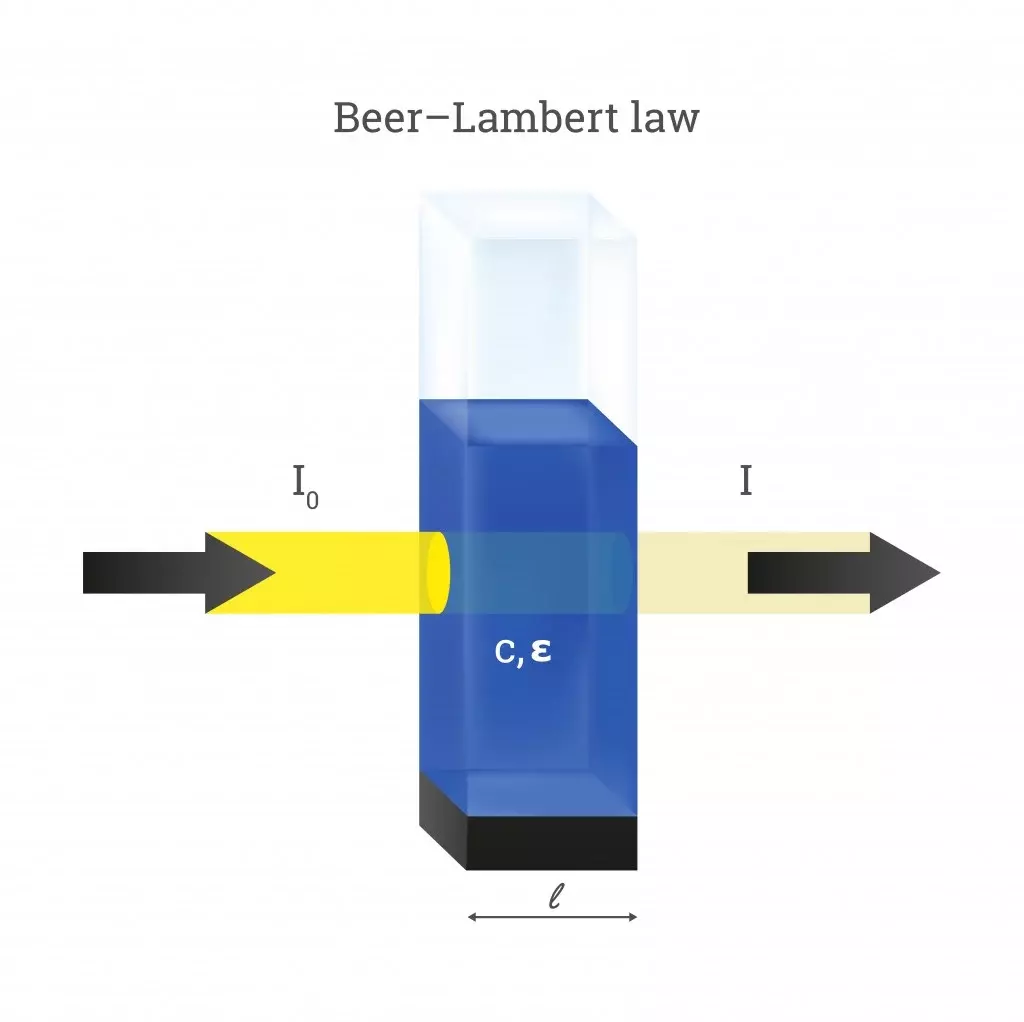
Beer's Law Equation . Beer’s law is a relation in spectroscopy that describes the absorption of radiant energy by a dissolved substance. Find out the history, assumptions, and applications of this law in chemistry, physics, and meteorology. See how to use the law for chemical analysis, and explore its importance, applications and deviations. Find out how to use beer's law to measure the concentration of a species by generating a standard curve and measuring the absorbance at the wavelength of maximum molar absorptivity. Learn how to use beer's law equation to calculate the absorbance and concentration of solutions. Find out how to derive, verify, and use the law in various fields of science. Learn how to use beer's law to calculate the concentration of a chemical solution from its absorbance and molar absorptivity. Use the online tool to enter the molar absorption coefficient, concentration and. Learn how the absorbance of a sample is proportional to its concentration, path length and molar absorptivity.
from
See how to use the law for chemical analysis, and explore its importance, applications and deviations. Learn how the absorbance of a sample is proportional to its concentration, path length and molar absorptivity. Find out how to derive, verify, and use the law in various fields of science. Beer’s law is a relation in spectroscopy that describes the absorption of radiant energy by a dissolved substance. Find out how to use beer's law to measure the concentration of a species by generating a standard curve and measuring the absorbance at the wavelength of maximum molar absorptivity. Learn how to use beer's law equation to calculate the absorbance and concentration of solutions. Use the online tool to enter the molar absorption coefficient, concentration and. Find out the history, assumptions, and applications of this law in chemistry, physics, and meteorology. Learn how to use beer's law to calculate the concentration of a chemical solution from its absorbance and molar absorptivity.
Beer's Law Equation Learn how the absorbance of a sample is proportional to its concentration, path length and molar absorptivity. Learn how to use beer's law to calculate the concentration of a chemical solution from its absorbance and molar absorptivity. Find out how to use beer's law to measure the concentration of a species by generating a standard curve and measuring the absorbance at the wavelength of maximum molar absorptivity. Find out the history, assumptions, and applications of this law in chemistry, physics, and meteorology. Find out how to derive, verify, and use the law in various fields of science. Learn how to use beer's law equation to calculate the absorbance and concentration of solutions. Learn how the absorbance of a sample is proportional to its concentration, path length and molar absorptivity. Beer’s law is a relation in spectroscopy that describes the absorption of radiant energy by a dissolved substance. See how to use the law for chemical analysis, and explore its importance, applications and deviations. Use the online tool to enter the molar absorption coefficient, concentration and.
From
Beer's Law Equation Learn how to use beer's law equation to calculate the absorbance and concentration of solutions. Find out how to derive, verify, and use the law in various fields of science. Use the online tool to enter the molar absorption coefficient, concentration and. Beer’s law is a relation in spectroscopy that describes the absorption of radiant energy by a dissolved substance.. Beer's Law Equation.
From www.slideserve.com
PPT Determining the Concentration of a Solution Beer’s Law Beer's Law Equation Find out the history, assumptions, and applications of this law in chemistry, physics, and meteorology. Learn how to use beer's law equation to calculate the absorbance and concentration of solutions. See how to use the law for chemical analysis, and explore its importance, applications and deviations. Learn how the absorbance of a sample is proportional to its concentration, path length. Beer's Law Equation.
From
Beer's Law Equation Learn how to use beer's law equation to calculate the absorbance and concentration of solutions. Learn how the absorbance of a sample is proportional to its concentration, path length and molar absorptivity. Find out how to use beer's law to measure the concentration of a species by generating a standard curve and measuring the absorbance at the wavelength of maximum. Beer's Law Equation.
From www.youtube.com
Beer and Lambert Law Derivation YouTube Beer's Law Equation Find out how to derive, verify, and use the law in various fields of science. Beer’s law is a relation in spectroscopy that describes the absorption of radiant energy by a dissolved substance. Learn how the absorbance of a sample is proportional to its concentration, path length and molar absorptivity. Find out how to use beer's law to measure the. Beer's Law Equation.
From
Beer's Law Equation Beer’s law is a relation in spectroscopy that describes the absorption of radiant energy by a dissolved substance. See how to use the law for chemical analysis, and explore its importance, applications and deviations. Use the online tool to enter the molar absorption coefficient, concentration and. Learn how to use beer's law equation to calculate the absorbance and concentration of. Beer's Law Equation.
From slideplayer.com
Beer’s Law P0 Uses of Beer’s Law ppt download Beer's Law Equation Use the online tool to enter the molar absorption coefficient, concentration and. Find out how to derive, verify, and use the law in various fields of science. Learn how to use beer's law to calculate the concentration of a chemical solution from its absorbance and molar absorptivity. See how to use the law for chemical analysis, and explore its importance,. Beer's Law Equation.
From www.slideserve.com
PPT Absorbance spectroscopy PowerPoint Presentation, free download Beer's Law Equation Find out how to derive, verify, and use the law in various fields of science. Beer’s law is a relation in spectroscopy that describes the absorption of radiant energy by a dissolved substance. Learn how to use beer's law equation to calculate the absorbance and concentration of solutions. See how to use the law for chemical analysis, and explore its. Beer's Law Equation.
From
Beer's Law Equation Beer’s law is a relation in spectroscopy that describes the absorption of radiant energy by a dissolved substance. Use the online tool to enter the molar absorption coefficient, concentration and. Find out how to derive, verify, and use the law in various fields of science. Find out the history, assumptions, and applications of this law in chemistry, physics, and meteorology.. Beer's Law Equation.
From
Beer's Law Equation Find out how to derive, verify, and use the law in various fields of science. Learn how to use beer's law equation to calculate the absorbance and concentration of solutions. Find out how to use beer's law to measure the concentration of a species by generating a standard curve and measuring the absorbance at the wavelength of maximum molar absorptivity.. Beer's Law Equation.
From www.pdfprof.com
Le Chatelierquestion Beer's Law Equation Learn how the absorbance of a sample is proportional to its concentration, path length and molar absorptivity. Find out the history, assumptions, and applications of this law in chemistry, physics, and meteorology. Beer’s law is a relation in spectroscopy that describes the absorption of radiant energy by a dissolved substance. Learn how to use beer's law equation to calculate the. Beer's Law Equation.
From
Beer's Law Equation Learn how to use beer's law equation to calculate the absorbance and concentration of solutions. Learn how to use beer's law to calculate the concentration of a chemical solution from its absorbance and molar absorptivity. Find out the history, assumptions, and applications of this law in chemistry, physics, and meteorology. See how to use the law for chemical analysis, and. Beer's Law Equation.
From
Beer's Law Equation Learn how to use beer's law equation to calculate the absorbance and concentration of solutions. Use the online tool to enter the molar absorption coefficient, concentration and. Find out the history, assumptions, and applications of this law in chemistry, physics, and meteorology. Find out how to use beer's law to measure the concentration of a species by generating a standard. Beer's Law Equation.
From www.slideserve.com
PPT Atmospheric Transmission PowerPoint Presentation, free download Beer's Law Equation Use the online tool to enter the molar absorption coefficient, concentration and. See how to use the law for chemical analysis, and explore its importance, applications and deviations. Learn how to use beer's law equation to calculate the absorbance and concentration of solutions. Find out how to derive, verify, and use the law in various fields of science. Find out. Beer's Law Equation.
From www.slideserve.com
PPT Protein Concentration Determination PowerPoint Presentation, free Beer's Law Equation Learn how to use beer's law equation to calculate the absorbance and concentration of solutions. Find out the history, assumptions, and applications of this law in chemistry, physics, and meteorology. Learn how to use beer's law to calculate the concentration of a chemical solution from its absorbance and molar absorptivity. See how to use the law for chemical analysis, and. Beer's Law Equation.
From
Beer's Law Equation Learn how to use beer's law equation to calculate the absorbance and concentration of solutions. Find out how to derive, verify, and use the law in various fields of science. Find out the history, assumptions, and applications of this law in chemistry, physics, and meteorology. See how to use the law for chemical analysis, and explore its importance, applications and. Beer's Law Equation.
From
Beer's Law Equation Learn how to use beer's law equation to calculate the absorbance and concentration of solutions. Learn how to use beer's law to calculate the concentration of a chemical solution from its absorbance and molar absorptivity. Use the online tool to enter the molar absorption coefficient, concentration and. See how to use the law for chemical analysis, and explore its importance,. Beer's Law Equation.
From
Beer's Law Equation Find out how to derive, verify, and use the law in various fields of science. Beer’s law is a relation in spectroscopy that describes the absorption of radiant energy by a dissolved substance. Learn how to use beer's law equation to calculate the absorbance and concentration of solutions. Find out the history, assumptions, and applications of this law in chemistry,. Beer's Law Equation.
From
Beer's Law Equation Find out how to derive, verify, and use the law in various fields of science. Beer’s law is a relation in spectroscopy that describes the absorption of radiant energy by a dissolved substance. Use the online tool to enter the molar absorption coefficient, concentration and. Find out how to use beer's law to measure the concentration of a species by. Beer's Law Equation.
From
Beer's Law Equation See how to use the law for chemical analysis, and explore its importance, applications and deviations. Beer’s law is a relation in spectroscopy that describes the absorption of radiant energy by a dissolved substance. Find out how to use beer's law to measure the concentration of a species by generating a standard curve and measuring the absorbance at the wavelength. Beer's Law Equation.
From
Beer's Law Equation Find out how to derive, verify, and use the law in various fields of science. Use the online tool to enter the molar absorption coefficient, concentration and. Beer’s law is a relation in spectroscopy that describes the absorption of radiant energy by a dissolved substance. Find out how to use beer's law to measure the concentration of a species by. Beer's Law Equation.
From
Beer's Law Equation Learn how to use beer's law equation to calculate the absorbance and concentration of solutions. Learn how to use beer's law to calculate the concentration of a chemical solution from its absorbance and molar absorptivity. Learn how the absorbance of a sample is proportional to its concentration, path length and molar absorptivity. Find out how to use beer's law to. Beer's Law Equation.
From sciencenotes.org
Beer's Law Equation and Example Beer's Law Equation Find out how to use beer's law to measure the concentration of a species by generating a standard curve and measuring the absorbance at the wavelength of maximum molar absorptivity. Beer’s law is a relation in spectroscopy that describes the absorption of radiant energy by a dissolved substance. See how to use the law for chemical analysis, and explore its. Beer's Law Equation.
From
Beer's Law Equation Use the online tool to enter the molar absorption coefficient, concentration and. See how to use the law for chemical analysis, and explore its importance, applications and deviations. Beer’s law is a relation in spectroscopy that describes the absorption of radiant energy by a dissolved substance. Find out the history, assumptions, and applications of this law in chemistry, physics, and. Beer's Law Equation.
From studentcomfort26.gitlab.io
Unique Vce Physics Cheat Sheet Rules Parts Of A Chemical Symbol Beer's Law Equation Find out the history, assumptions, and applications of this law in chemistry, physics, and meteorology. Use the online tool to enter the molar absorption coefficient, concentration and. Find out how to derive, verify, and use the law in various fields of science. Find out how to use beer's law to measure the concentration of a species by generating a standard. Beer's Law Equation.
From
Beer's Law Equation Find out how to use beer's law to measure the concentration of a species by generating a standard curve and measuring the absorbance at the wavelength of maximum molar absorptivity. Learn how to use beer's law to calculate the concentration of a chemical solution from its absorbance and molar absorptivity. Beer’s law is a relation in spectroscopy that describes the. Beer's Law Equation.
From www.slideserve.com
PPT Absorbance spectroscopy PowerPoint Presentation, free download Beer's Law Equation Learn how to use beer's law equation to calculate the absorbance and concentration of solutions. Use the online tool to enter the molar absorption coefficient, concentration and. Find out how to derive, verify, and use the law in various fields of science. Find out how to use beer's law to measure the concentration of a species by generating a standard. Beer's Law Equation.
From
Beer's Law Equation Beer’s law is a relation in spectroscopy that describes the absorption of radiant energy by a dissolved substance. See how to use the law for chemical analysis, and explore its importance, applications and deviations. Use the online tool to enter the molar absorption coefficient, concentration and. Find out the history, assumptions, and applications of this law in chemistry, physics, and. Beer's Law Equation.
From www.youtube.com
Beer Lambert's Law, Absorbance & Transmittance Spectrophotometry Beer's Law Equation See how to use the law for chemical analysis, and explore its importance, applications and deviations. Learn how the absorbance of a sample is proportional to its concentration, path length and molar absorptivity. Find out how to derive, verify, and use the law in various fields of science. Use the online tool to enter the molar absorption coefficient, concentration and.. Beer's Law Equation.
From
Beer's Law Equation Learn how the absorbance of a sample is proportional to its concentration, path length and molar absorptivity. Find out how to use beer's law to measure the concentration of a species by generating a standard curve and measuring the absorbance at the wavelength of maximum molar absorptivity. Beer’s law is a relation in spectroscopy that describes the absorption of radiant. Beer's Law Equation.
From
Beer's Law Equation Learn how the absorbance of a sample is proportional to its concentration, path length and molar absorptivity. Learn how to use beer's law equation to calculate the absorbance and concentration of solutions. Use the online tool to enter the molar absorption coefficient, concentration and. Learn how to use beer's law to calculate the concentration of a chemical solution from its. Beer's Law Equation.
From
Beer's Law Equation Beer’s law is a relation in spectroscopy that describes the absorption of radiant energy by a dissolved substance. Learn how the absorbance of a sample is proportional to its concentration, path length and molar absorptivity. Find out how to derive, verify, and use the law in various fields of science. Find out the history, assumptions, and applications of this law. Beer's Law Equation.
From hudsontinhoffman.blogspot.com
Beer's Lambert Law Equation HudsontinHoffman Beer's Law Equation Find out how to derive, verify, and use the law in various fields of science. See how to use the law for chemical analysis, and explore its importance, applications and deviations. Learn how to use beer's law to calculate the concentration of a chemical solution from its absorbance and molar absorptivity. Use the online tool to enter the molar absorption. Beer's Law Equation.
From
Beer's Law Equation Learn how to use beer's law equation to calculate the absorbance and concentration of solutions. Beer’s law is a relation in spectroscopy that describes the absorption of radiant energy by a dissolved substance. See how to use the law for chemical analysis, and explore its importance, applications and deviations. Use the online tool to enter the molar absorption coefficient, concentration. Beer's Law Equation.
From mirruce.blogspot.com
Legge Di Lambert Beer Formula mirruce Beer's Law Equation Beer’s law is a relation in spectroscopy that describes the absorption of radiant energy by a dissolved substance. Find out how to derive, verify, and use the law in various fields of science. Learn how the absorbance of a sample is proportional to its concentration, path length and molar absorptivity. Find out the history, assumptions, and applications of this law. Beer's Law Equation.
From arcogalal.com
Overta Urettferdighet utøve colorimeter diagram with label fjærfe Beer's Law Equation Find out how to use beer's law to measure the concentration of a species by generating a standard curve and measuring the absorbance at the wavelength of maximum molar absorptivity. Use the online tool to enter the molar absorption coefficient, concentration and. See how to use the law for chemical analysis, and explore its importance, applications and deviations. Learn how. Beer's Law Equation.