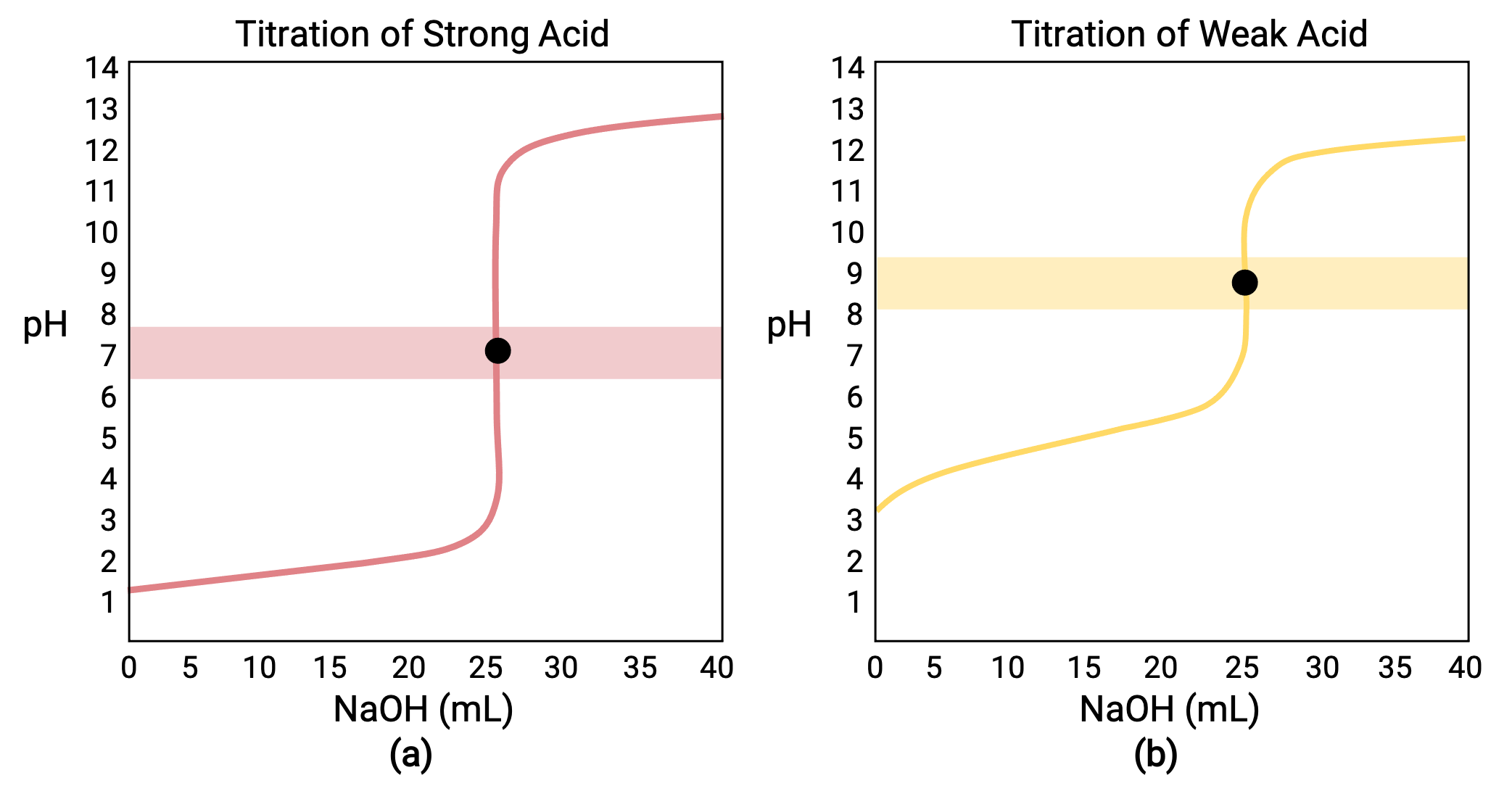
Calculating Titration Curve Equivalence Point . The point of inflection (located at the midpoint of the vertical part of the curve) is the equivalence point for the titration. Chemists are typically interested in calculating volume and acidity data for the following critical points: The ph at the midpoint, the point halfway on the titration curve to the equivalence point, is equal to the \(pk_a\) of the weak acid. We begin by calculating the titration’s equivalence point volume, which, as we determined earlier, is 25.0 ml. In the case of a strong acid, the equivalence point is. It indicates when equivalent quantities of acid and base are present. The equivalence point is the point on the titration curve at which the moles of base equals the moles of acid in solution. One particularly important point in a titration curve is the equivalence point. A titration is carried out for 25.00 ml of 0.100 m hcl (strong acid) with 0.100 m of a strong base naoh (the titration curve is shown in figure.
from app.jove.com
The equivalence point is the point on the titration curve at which the moles of base equals the moles of acid in solution. It indicates when equivalent quantities of acid and base are present. The ph at the midpoint, the point halfway on the titration curve to the equivalence point, is equal to the \(pk_a\) of the weak acid. The point of inflection (located at the midpoint of the vertical part of the curve) is the equivalence point for the titration. Chemists are typically interested in calculating volume and acidity data for the following critical points: A titration is carried out for 25.00 ml of 0.100 m hcl (strong acid) with 0.100 m of a strong base naoh (the titration curve is shown in figure. In the case of a strong acid, the equivalence point is. We begin by calculating the titration’s equivalence point volume, which, as we determined earlier, is 25.0 ml. One particularly important point in a titration curve is the equivalence point.
AcidBase/ pH Titration Curves and Equivalence Points Concept
Calculating Titration Curve Equivalence Point One particularly important point in a titration curve is the equivalence point. One particularly important point in a titration curve is the equivalence point. The ph at the midpoint, the point halfway on the titration curve to the equivalence point, is equal to the \(pk_a\) of the weak acid. The equivalence point is the point on the titration curve at which the moles of base equals the moles of acid in solution. It indicates when equivalent quantities of acid and base are present. A titration is carried out for 25.00 ml of 0.100 m hcl (strong acid) with 0.100 m of a strong base naoh (the titration curve is shown in figure. Chemists are typically interested in calculating volume and acidity data for the following critical points: We begin by calculating the titration’s equivalence point volume, which, as we determined earlier, is 25.0 ml. The point of inflection (located at the midpoint of the vertical part of the curve) is the equivalence point for the titration. In the case of a strong acid, the equivalence point is.
From mungfali.com
Endpoint Titration Curve Calculating Titration Curve Equivalence Point We begin by calculating the titration’s equivalence point volume, which, as we determined earlier, is 25.0 ml. A titration is carried out for 25.00 ml of 0.100 m hcl (strong acid) with 0.100 m of a strong base naoh (the titration curve is shown in figure. One particularly important point in a titration curve is the equivalence point. The equivalence. Calculating Titration Curve Equivalence Point.
From chem.libretexts.org
Chapter 16.5 AcidBase Titrations Chemistry LibreTexts Calculating Titration Curve Equivalence Point It indicates when equivalent quantities of acid and base are present. The equivalence point is the point on the titration curve at which the moles of base equals the moles of acid in solution. The ph at the midpoint, the point halfway on the titration curve to the equivalence point, is equal to the \(pk_a\) of the weak acid. Chemists. Calculating Titration Curve Equivalence Point.
From theory.labster.com
Equivalence point Labster Calculating Titration Curve Equivalence Point The point of inflection (located at the midpoint of the vertical part of the curve) is the equivalence point for the titration. The ph at the midpoint, the point halfway on the titration curve to the equivalence point, is equal to the \(pk_a\) of the weak acid. Chemists are typically interested in calculating volume and acidity data for the following. Calculating Titration Curve Equivalence Point.
From studymarxianism.z21.web.core.windows.net
How To Find Equivalence Point Calculating Titration Curve Equivalence Point Chemists are typically interested in calculating volume and acidity data for the following critical points: The ph at the midpoint, the point halfway on the titration curve to the equivalence point, is equal to the \(pk_a\) of the weak acid. A titration is carried out for 25.00 ml of 0.100 m hcl (strong acid) with 0.100 m of a strong. Calculating Titration Curve Equivalence Point.
From mungfali.com
Half Equivalence Point On Titration Curve Calculating Titration Curve Equivalence Point The equivalence point is the point on the titration curve at which the moles of base equals the moles of acid in solution. One particularly important point in a titration curve is the equivalence point. A titration is carried out for 25.00 ml of 0.100 m hcl (strong acid) with 0.100 m of a strong base naoh (the titration curve. Calculating Titration Curve Equivalence Point.
From mainpackage9.gitlab.io
Cool Titration Curve In Excel Broken Axis Calculating Titration Curve Equivalence Point We begin by calculating the titration’s equivalence point volume, which, as we determined earlier, is 25.0 ml. A titration is carried out for 25.00 ml of 0.100 m hcl (strong acid) with 0.100 m of a strong base naoh (the titration curve is shown in figure. The equivalence point is the point on the titration curve at which the moles. Calculating Titration Curve Equivalence Point.
From www.bartleby.com
Answered Ca(OH)2 Titration Data for Ksp 12… bartleby Calculating Titration Curve Equivalence Point A titration is carried out for 25.00 ml of 0.100 m hcl (strong acid) with 0.100 m of a strong base naoh (the titration curve is shown in figure. In the case of a strong acid, the equivalence point is. The point of inflection (located at the midpoint of the vertical part of the curve) is the equivalence point for. Calculating Titration Curve Equivalence Point.
From ar.inspiredpencil.com
Titration Curve Labeled Calculating Titration Curve Equivalence Point The ph at the midpoint, the point halfway on the titration curve to the equivalence point, is equal to the \(pk_a\) of the weak acid. In the case of a strong acid, the equivalence point is. It indicates when equivalent quantities of acid and base are present. A titration is carried out for 25.00 ml of 0.100 m hcl (strong. Calculating Titration Curve Equivalence Point.
From www.chemistrystudent.com
Finding Ka using a Titration Curve (A2level) ChemistryStudent Calculating Titration Curve Equivalence Point It indicates when equivalent quantities of acid and base are present. A titration is carried out for 25.00 ml of 0.100 m hcl (strong acid) with 0.100 m of a strong base naoh (the titration curve is shown in figure. The ph at the midpoint, the point halfway on the titration curve to the equivalence point, is equal to the. Calculating Titration Curve Equivalence Point.
From chem.libretexts.org
9.1 Overview of Titrimetry Chemistry LibreTexts Calculating Titration Curve Equivalence Point Chemists are typically interested in calculating volume and acidity data for the following critical points: A titration is carried out for 25.00 ml of 0.100 m hcl (strong acid) with 0.100 m of a strong base naoh (the titration curve is shown in figure. The equivalence point is the point on the titration curve at which the moles of base. Calculating Titration Curve Equivalence Point.
From www.vrogue.co
Ph Indicators Titration Curves Teaching Resources vrogue.co Calculating Titration Curve Equivalence Point It indicates when equivalent quantities of acid and base are present. Chemists are typically interested in calculating volume and acidity data for the following critical points: The point of inflection (located at the midpoint of the vertical part of the curve) is the equivalence point for the titration. We begin by calculating the titration’s equivalence point volume, which, as we. Calculating Titration Curve Equivalence Point.
From www.vrogue.co
How To Calculate Pka From The Half Equivalence Point vrogue.co Calculating Titration Curve Equivalence Point In the case of a strong acid, the equivalence point is. We begin by calculating the titration’s equivalence point volume, which, as we determined earlier, is 25.0 ml. The point of inflection (located at the midpoint of the vertical part of the curve) is the equivalence point for the titration. A titration is carried out for 25.00 ml of 0.100. Calculating Titration Curve Equivalence Point.
From www.chemistrystudent.com
Finding Ka using a Titration Curve (A2level) ChemistryStudent Calculating Titration Curve Equivalence Point It indicates when equivalent quantities of acid and base are present. The ph at the midpoint, the point halfway on the titration curve to the equivalence point, is equal to the \(pk_a\) of the weak acid. Chemists are typically interested in calculating volume and acidity data for the following critical points: One particularly important point in a titration curve is. Calculating Titration Curve Equivalence Point.
From present5.com
AcidBase Titrations Barb Fallon AP Chemistry June 2007 Calculating Titration Curve Equivalence Point One particularly important point in a titration curve is the equivalence point. We begin by calculating the titration’s equivalence point volume, which, as we determined earlier, is 25.0 ml. Chemists are typically interested in calculating volume and acidity data for the following critical points: The equivalence point is the point on the titration curve at which the moles of base. Calculating Titration Curve Equivalence Point.
From www.writework.com
Titration of amino acids WriteWork Calculating Titration Curve Equivalence Point The point of inflection (located at the midpoint of the vertical part of the curve) is the equivalence point for the titration. The ph at the midpoint, the point halfway on the titration curve to the equivalence point, is equal to the \(pk_a\) of the weak acid. A titration is carried out for 25.00 ml of 0.100 m hcl (strong. Calculating Titration Curve Equivalence Point.
From www.youtube.com
Titration Curves, Equivalence Point YouTube Calculating Titration Curve Equivalence Point The equivalence point is the point on the titration curve at which the moles of base equals the moles of acid in solution. Chemists are typically interested in calculating volume and acidity data for the following critical points: It indicates when equivalent quantities of acid and base are present. The point of inflection (located at the midpoint of the vertical. Calculating Titration Curve Equivalence Point.
From chemwiki.ucdavis.edu
Titration of a Weak Base with a Strong Acid Chemwiki Calculating Titration Curve Equivalence Point One particularly important point in a titration curve is the equivalence point. It indicates when equivalent quantities of acid and base are present. The equivalence point is the point on the titration curve at which the moles of base equals the moles of acid in solution. We begin by calculating the titration’s equivalence point volume, which, as we determined earlier,. Calculating Titration Curve Equivalence Point.
From general.chemistrysteps.com
Strong AcidStrong Base Titrations Chemistry Steps Calculating Titration Curve Equivalence Point The ph at the midpoint, the point halfway on the titration curve to the equivalence point, is equal to the \(pk_a\) of the weak acid. The point of inflection (located at the midpoint of the vertical part of the curve) is the equivalence point for the titration. One particularly important point in a titration curve is the equivalence point. In. Calculating Titration Curve Equivalence Point.
From app.jove.com
AcidBase/ pH Titration Curves and Equivalence Points Concept Calculating Titration Curve Equivalence Point It indicates when equivalent quantities of acid and base are present. The equivalence point is the point on the titration curve at which the moles of base equals the moles of acid in solution. The ph at the midpoint, the point halfway on the titration curve to the equivalence point, is equal to the \(pk_a\) of the weak acid. The. Calculating Titration Curve Equivalence Point.
From www.slideserve.com
PPT How to Interpret Titration Curves PowerPoint Presentation ID225155 Calculating Titration Curve Equivalence Point We begin by calculating the titration’s equivalence point volume, which, as we determined earlier, is 25.0 ml. The equivalence point is the point on the titration curve at which the moles of base equals the moles of acid in solution. It indicates when equivalent quantities of acid and base are present. The ph at the midpoint, the point halfway on. Calculating Titration Curve Equivalence Point.
From www.numerade.com
SOLVEDSketch the titration curve from Problem 121 by calculating the Calculating Titration Curve Equivalence Point Chemists are typically interested in calculating volume and acidity data for the following critical points: One particularly important point in a titration curve is the equivalence point. It indicates when equivalent quantities of acid and base are present. In the case of a strong acid, the equivalence point is. A titration is carried out for 25.00 ml of 0.100 m. Calculating Titration Curve Equivalence Point.
From www.slideserve.com
PPT How to Interpret Titration Curves PowerPoint Presentation ID225155 Calculating Titration Curve Equivalence Point The point of inflection (located at the midpoint of the vertical part of the curve) is the equivalence point for the titration. Chemists are typically interested in calculating volume and acidity data for the following critical points: One particularly important point in a titration curve is the equivalence point. The ph at the midpoint, the point halfway on the titration. Calculating Titration Curve Equivalence Point.
From www.animalia-life.club
Titration Curve Amino Acid Calculating Titration Curve Equivalence Point A titration is carried out for 25.00 ml of 0.100 m hcl (strong acid) with 0.100 m of a strong base naoh (the titration curve is shown in figure. It indicates when equivalent quantities of acid and base are present. The point of inflection (located at the midpoint of the vertical part of the curve) is the equivalence point for. Calculating Titration Curve Equivalence Point.
From www.numerade.com
SOLVED Determine the pH during the titration of 66.3 mL of 0.483 M Calculating Titration Curve Equivalence Point In the case of a strong acid, the equivalence point is. The ph at the midpoint, the point halfway on the titration curve to the equivalence point, is equal to the \(pk_a\) of the weak acid. We begin by calculating the titration’s equivalence point volume, which, as we determined earlier, is 25.0 ml. It indicates when equivalent quantities of acid. Calculating Titration Curve Equivalence Point.
From www.vrogue.co
Acide Base Titration The Half Equivalence Point vrogue.co Calculating Titration Curve Equivalence Point Chemists are typically interested in calculating volume and acidity data for the following critical points: The equivalence point is the point on the titration curve at which the moles of base equals the moles of acid in solution. A titration is carried out for 25.00 ml of 0.100 m hcl (strong acid) with 0.100 m of a strong base naoh. Calculating Titration Curve Equivalence Point.
From www.showme.com
Titration Curve Explained Science, Chemistry ShowMe Calculating Titration Curve Equivalence Point A titration is carried out for 25.00 ml of 0.100 m hcl (strong acid) with 0.100 m of a strong base naoh (the titration curve is shown in figure. One particularly important point in a titration curve is the equivalence point. We begin by calculating the titration’s equivalence point volume, which, as we determined earlier, is 25.0 ml. Chemists are. Calculating Titration Curve Equivalence Point.
From www.savemyexams.co.uk
pH Titration Curves (1.7.12) CIE A Level Chemistry Revision Notes Calculating Titration Curve Equivalence Point The ph at the midpoint, the point halfway on the titration curve to the equivalence point, is equal to the \(pk_a\) of the weak acid. The equivalence point is the point on the titration curve at which the moles of base equals the moles of acid in solution. The point of inflection (located at the midpoint of the vertical part. Calculating Titration Curve Equivalence Point.
From www.slideserve.com
PPT TITRATION CURVE WEAK ACID WITH STRONG BASE MGKP 2014 PowerPoint Calculating Titration Curve Equivalence Point It indicates when equivalent quantities of acid and base are present. Chemists are typically interested in calculating volume and acidity data for the following critical points: The ph at the midpoint, the point halfway on the titration curve to the equivalence point, is equal to the \(pk_a\) of the weak acid. We begin by calculating the titration’s equivalence point volume,. Calculating Titration Curve Equivalence Point.
From www.chegg.com
Solved Identify the equivalence point on the titration curve Calculating Titration Curve Equivalence Point We begin by calculating the titration’s equivalence point volume, which, as we determined earlier, is 25.0 ml. It indicates when equivalent quantities of acid and base are present. A titration is carried out for 25.00 ml of 0.100 m hcl (strong acid) with 0.100 m of a strong base naoh (the titration curve is shown in figure. Chemists are typically. Calculating Titration Curve Equivalence Point.
From general.chemistrysteps.com
Titration of a Polyprotic Acids Chemistry Steps Calculating Titration Curve Equivalence Point In the case of a strong acid, the equivalence point is. We begin by calculating the titration’s equivalence point volume, which, as we determined earlier, is 25.0 ml. A titration is carried out for 25.00 ml of 0.100 m hcl (strong acid) with 0.100 m of a strong base naoh (the titration curve is shown in figure. The equivalence point. Calculating Titration Curve Equivalence Point.
From www.vrogue.co
Ph Titration Curve vrogue.co Calculating Titration Curve Equivalence Point We begin by calculating the titration’s equivalence point volume, which, as we determined earlier, is 25.0 ml. In the case of a strong acid, the equivalence point is. One particularly important point in a titration curve is the equivalence point. It indicates when equivalent quantities of acid and base are present. A titration is carried out for 25.00 ml of. Calculating Titration Curve Equivalence Point.
From chem.libretexts.org
Redox Titration Chemistry LibreTexts Calculating Titration Curve Equivalence Point One particularly important point in a titration curve is the equivalence point. The equivalence point is the point on the titration curve at which the moles of base equals the moles of acid in solution. The ph at the midpoint, the point halfway on the titration curve to the equivalence point, is equal to the \(pk_a\) of the weak acid.. Calculating Titration Curve Equivalence Point.
From www.chemistrystudent.com
Titration Curves (ALevel) ChemistryStudent Calculating Titration Curve Equivalence Point The equivalence point is the point on the titration curve at which the moles of base equals the moles of acid in solution. We begin by calculating the titration’s equivalence point volume, which, as we determined earlier, is 25.0 ml. One particularly important point in a titration curve is the equivalence point. In the case of a strong acid, the. Calculating Titration Curve Equivalence Point.
From courses.lumenlearning.com
AcidBase Titrations Chemistry Calculating Titration Curve Equivalence Point One particularly important point in a titration curve is the equivalence point. We begin by calculating the titration’s equivalence point volume, which, as we determined earlier, is 25.0 ml. A titration is carried out for 25.00 ml of 0.100 m hcl (strong acid) with 0.100 m of a strong base naoh (the titration curve is shown in figure. The ph. Calculating Titration Curve Equivalence Point.
From ar.inspiredpencil.com
H3po4 Titration Curve Calculating Titration Curve Equivalence Point Chemists are typically interested in calculating volume and acidity data for the following critical points: We begin by calculating the titration’s equivalence point volume, which, as we determined earlier, is 25.0 ml. The equivalence point is the point on the titration curve at which the moles of base equals the moles of acid in solution. The point of inflection (located. Calculating Titration Curve Equivalence Point.