Lead Carbonate And Nitric Acid . The balanced equation will be calculated along with the. The equation for this reaction is:. We were trying to dissolve a piece of metallic lead in concentrated nitric acid until we found out that concentrated nitric acid is not a. (b) a solution of lead(ii) nitrate can be made by reacting solid lead(ii) carbonate with dilute nitric acid. The equation for this reaction is: Lead reacts slowly nitric acid, $\ce{hno3}$. According to the crc handbook of chemistry and physics 41st edition, lead dissolves in nitric acid. This page looks at the formation of some insoluble lead(ii) compounds from aqueous lead(ii) ions using precipitation reactions. Enter an equation of an ionic chemical equation and press the balance button. When acids react with carbonates, such as calcium carbonate (found in chalk, limestone and marble), a salt, water and carbon dioxide are. Pbco 3(s) + 2hno 3(aq) → pb(no 3). As is the case with other unreactive. Nitrogen oxides are formed together with lead(ii) nitrate, $\ce{pb(no3)2}$. (1 point) a solution of lead(ii) nitrate can be made by reacting solid lead(ii) carbonate with dilute acid.
from www.masterorganicchemistry.com
(1 point) a solution of lead(ii) nitrate can be made by reacting solid lead(ii) carbonate with dilute acid. The equation for this reaction is:. Enter an equation of an ionic chemical equation and press the balance button. This page looks at the formation of some insoluble lead(ii) compounds from aqueous lead(ii) ions using precipitation reactions. Nitrogen oxides are formed together with lead(ii) nitrate, $\ce{pb(no3)2}$. When acids react with carbonates, such as calcium carbonate (found in chalk, limestone and marble), a salt, water and carbon dioxide are. The equation for this reaction is: The balanced equation will be calculated along with the. Lead reacts slowly nitric acid, $\ce{hno3}$. As is the case with other unreactive.
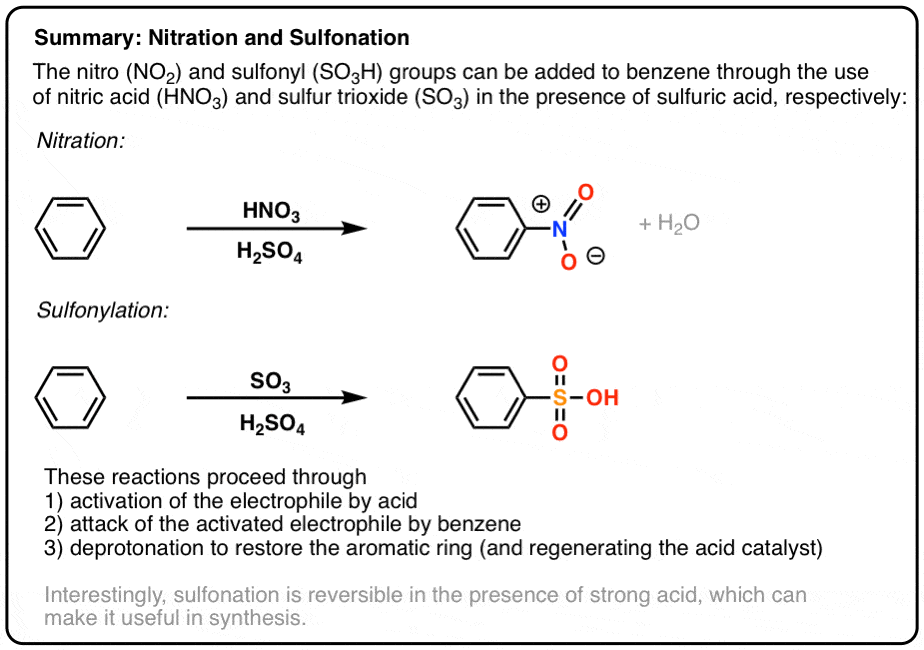
Nitration and Sulfonation Reactions In Electrophilic Aromatic Substitution
Lead Carbonate And Nitric Acid The equation for this reaction is: Nitrogen oxides are formed together with lead(ii) nitrate, $\ce{pb(no3)2}$. When acids react with carbonates, such as calcium carbonate (found in chalk, limestone and marble), a salt, water and carbon dioxide are. Pbco 3(s) + 2hno 3(aq) → pb(no 3). (1 point) a solution of lead(ii) nitrate can be made by reacting solid lead(ii) carbonate with dilute acid. Lead reacts slowly nitric acid, $\ce{hno3}$. According to the crc handbook of chemistry and physics 41st edition, lead dissolves in nitric acid. As is the case with other unreactive. (b) a solution of lead(ii) nitrate can be made by reacting solid lead(ii) carbonate with dilute nitric acid. The equation for this reaction is:. The balanced equation will be calculated along with the. Enter an equation of an ionic chemical equation and press the balance button. The equation for this reaction is: This page looks at the formation of some insoluble lead(ii) compounds from aqueous lead(ii) ions using precipitation reactions. We were trying to dissolve a piece of metallic lead in concentrated nitric acid until we found out that concentrated nitric acid is not a.
From www.dreamstime.com
Nitric Acid Carbonate Chemical Formula on Waterdrop Background Stock Lead Carbonate And Nitric Acid Nitrogen oxides are formed together with lead(ii) nitrate, $\ce{pb(no3)2}$. Enter an equation of an ionic chemical equation and press the balance button. We were trying to dissolve a piece of metallic lead in concentrated nitric acid until we found out that concentrated nitric acid is not a. When acids react with carbonates, such as calcium carbonate (found in chalk, limestone. Lead Carbonate And Nitric Acid.
From www.youtube.com
Testing of Iodide Anions _ lead(II) nitrate and nitric acid YouTube Lead Carbonate And Nitric Acid Nitrogen oxides are formed together with lead(ii) nitrate, $\ce{pb(no3)2}$. Pbco 3(s) + 2hno 3(aq) → pb(no 3). The equation for this reaction is: As is the case with other unreactive. Enter an equation of an ionic chemical equation and press the balance button. According to the crc handbook of chemistry and physics 41st edition, lead dissolves in nitric acid. The. Lead Carbonate And Nitric Acid.
From spmchemistry.blog.onlinetuition.com.my
Acid + Carbonate SPM Chemistry Lead Carbonate And Nitric Acid This page looks at the formation of some insoluble lead(ii) compounds from aqueous lead(ii) ions using precipitation reactions. Lead reacts slowly nitric acid, $\ce{hno3}$. We were trying to dissolve a piece of metallic lead in concentrated nitric acid until we found out that concentrated nitric acid is not a. According to the crc handbook of chemistry and physics 41st edition,. Lead Carbonate And Nitric Acid.
From www.vrogue.co
Nitric Acid Reacts With Calcium Carbonate Balanced Eq vrogue.co Lead Carbonate And Nitric Acid Enter an equation of an ionic chemical equation and press the balance button. We were trying to dissolve a piece of metallic lead in concentrated nitric acid until we found out that concentrated nitric acid is not a. According to the crc handbook of chemistry and physics 41st edition, lead dissolves in nitric acid. Nitrogen oxides are formed together with. Lead Carbonate And Nitric Acid.
From www.onlinemathlearning.com
Writing Ionic Equation (video lessons, examples and solutions) Lead Carbonate And Nitric Acid According to the crc handbook of chemistry and physics 41st edition, lead dissolves in nitric acid. (b) a solution of lead(ii) nitrate can be made by reacting solid lead(ii) carbonate with dilute nitric acid. The equation for this reaction is:. When acids react with carbonates, such as calcium carbonate (found in chalk, limestone and marble), a salt, water and carbon. Lead Carbonate And Nitric Acid.
From www.youtube.com
The Reaction Between Lead (II) Nitrate and Sodium Carbonate YouTube Lead Carbonate And Nitric Acid Pbco 3(s) + 2hno 3(aq) → pb(no 3). The equation for this reaction is: Enter an equation of an ionic chemical equation and press the balance button. When acids react with carbonates, such as calcium carbonate (found in chalk, limestone and marble), a salt, water and carbon dioxide are. (1 point) a solution of lead(ii) nitrate can be made by. Lead Carbonate And Nitric Acid.
From www.youtube.com
sodium carbonate and nitric acid total ionic equation YouTube Lead Carbonate And Nitric Acid Enter an equation of an ionic chemical equation and press the balance button. The equation for this reaction is:. According to the crc handbook of chemistry and physics 41st edition, lead dissolves in nitric acid. (1 point) a solution of lead(ii) nitrate can be made by reacting solid lead(ii) carbonate with dilute acid. This page looks at the formation of. Lead Carbonate And Nitric Acid.
From wisc.pb.unizin.org
Acids, Bases, Neutralization, and GasForming Reactions (M3Q34) UW Lead Carbonate And Nitric Acid We were trying to dissolve a piece of metallic lead in concentrated nitric acid until we found out that concentrated nitric acid is not a. (1 point) a solution of lead(ii) nitrate can be made by reacting solid lead(ii) carbonate with dilute acid. As is the case with other unreactive. When acids react with carbonates, such as calcium carbonate (found. Lead Carbonate And Nitric Acid.
From www.chegg.com
Solved When magnesium carbonate is added to nitric acid, Lead Carbonate And Nitric Acid (b) a solution of lead(ii) nitrate can be made by reacting solid lead(ii) carbonate with dilute nitric acid. As is the case with other unreactive. Enter an equation of an ionic chemical equation and press the balance button. According to the crc handbook of chemistry and physics 41st edition, lead dissolves in nitric acid. Pbco 3(s) + 2hno 3(aq) →. Lead Carbonate And Nitric Acid.
From www.showme.com
ShowMe sodium carbonate and nitric acid Lead Carbonate And Nitric Acid Enter an equation of an ionic chemical equation and press the balance button. When acids react with carbonates, such as calcium carbonate (found in chalk, limestone and marble), a salt, water and carbon dioxide are. This page looks at the formation of some insoluble lead(ii) compounds from aqueous lead(ii) ions using precipitation reactions. The equation for this reaction is:. (1. Lead Carbonate And Nitric Acid.
From www.youtube.com
Write the balanced chemical equation of the following word equation Lead Carbonate And Nitric Acid The balanced equation will be calculated along with the. Nitrogen oxides are formed together with lead(ii) nitrate, $\ce{pb(no3)2}$. (b) a solution of lead(ii) nitrate can be made by reacting solid lead(ii) carbonate with dilute nitric acid. The equation for this reaction is:. Enter an equation of an ionic chemical equation and press the balance button. Pbco 3(s) + 2hno 3(aq). Lead Carbonate And Nitric Acid.
From www.masterorganicchemistry.com
Nitration and Sulfonation Reactions In Electrophilic Aromatic Substitution Lead Carbonate And Nitric Acid The equation for this reaction is: This page looks at the formation of some insoluble lead(ii) compounds from aqueous lead(ii) ions using precipitation reactions. (b) a solution of lead(ii) nitrate can be made by reacting solid lead(ii) carbonate with dilute nitric acid. (1 point) a solution of lead(ii) nitrate can be made by reacting solid lead(ii) carbonate with dilute acid.. Lead Carbonate And Nitric Acid.
From www.youtube.com
Making Salts From Acids & Metal Carbonates (GCSE Chemistry) YouTube Lead Carbonate And Nitric Acid When acids react with carbonates, such as calcium carbonate (found in chalk, limestone and marble), a salt, water and carbon dioxide are. Lead reacts slowly nitric acid, $\ce{hno3}$. Nitrogen oxides are formed together with lead(ii) nitrate, $\ce{pb(no3)2}$. According to the crc handbook of chemistry and physics 41st edition, lead dissolves in nitric acid. The balanced equation will be calculated along. Lead Carbonate And Nitric Acid.
From www.youtube.com
When zinc reacts with very dilute nitric acid it produces YouTube Lead Carbonate And Nitric Acid We were trying to dissolve a piece of metallic lead in concentrated nitric acid until we found out that concentrated nitric acid is not a. The equation for this reaction is:. The balanced equation will be calculated along with the. The equation for this reaction is: According to the crc handbook of chemistry and physics 41st edition, lead dissolves in. Lead Carbonate And Nitric Acid.
From www.chegg.com
Solved 3, lead(II) nitrate + potassium sulfate ? (NOs )2 4 Lead Carbonate And Nitric Acid The balanced equation will be calculated along with the. (b) a solution of lead(ii) nitrate can be made by reacting solid lead(ii) carbonate with dilute nitric acid. (1 point) a solution of lead(ii) nitrate can be made by reacting solid lead(ii) carbonate with dilute acid. This page looks at the formation of some insoluble lead(ii) compounds from aqueous lead(ii) ions. Lead Carbonate And Nitric Acid.
From www.numerade.com
SOLVED M02 Write the molecular; total ionic, and net ionic equation Lead Carbonate And Nitric Acid (1 point) a solution of lead(ii) nitrate can be made by reacting solid lead(ii) carbonate with dilute acid. When acids react with carbonates, such as calcium carbonate (found in chalk, limestone and marble), a salt, water and carbon dioxide are. This page looks at the formation of some insoluble lead(ii) compounds from aqueous lead(ii) ions using precipitation reactions. The equation. Lead Carbonate And Nitric Acid.
From www.showme.com
Ashok (sodium carbonate and nitric acid) total ionic equation Science Lead Carbonate And Nitric Acid As is the case with other unreactive. According to the crc handbook of chemistry and physics 41st edition, lead dissolves in nitric acid. The balanced equation will be calculated along with the. Lead reacts slowly nitric acid, $\ce{hno3}$. (b) a solution of lead(ii) nitrate can be made by reacting solid lead(ii) carbonate with dilute nitric acid. This page looks at. Lead Carbonate And Nitric Acid.
From studylib.net
Net Ionic Equations Lead Carbonate And Nitric Acid This page looks at the formation of some insoluble lead(ii) compounds from aqueous lead(ii) ions using precipitation reactions. When acids react with carbonates, such as calcium carbonate (found in chalk, limestone and marble), a salt, water and carbon dioxide are. The equation for this reaction is: Enter an equation of an ionic chemical equation and press the balance button. The. Lead Carbonate And Nitric Acid.
From www.chegg.com
Solved Nitric acid reacts with sodium carbonate to form Lead Carbonate And Nitric Acid (b) a solution of lead(ii) nitrate can be made by reacting solid lead(ii) carbonate with dilute nitric acid. This page looks at the formation of some insoluble lead(ii) compounds from aqueous lead(ii) ions using precipitation reactions. Enter an equation of an ionic chemical equation and press the balance button. When acids react with carbonates, such as calcium carbonate (found in. Lead Carbonate And Nitric Acid.
From www.pinterest.com
Test for a Carbonate infographic diagram showing a laboratory Lead Carbonate And Nitric Acid (b) a solution of lead(ii) nitrate can be made by reacting solid lead(ii) carbonate with dilute nitric acid. As is the case with other unreactive. Nitrogen oxides are formed together with lead(ii) nitrate, $\ce{pb(no3)2}$. The equation for this reaction is:. According to the crc handbook of chemistry and physics 41st edition, lead dissolves in nitric acid. The balanced equation will. Lead Carbonate And Nitric Acid.
From www.youtube.com
Make Nitric Acid from Sodium Bisulfate and Sodium Nitrate YouTube Lead Carbonate And Nitric Acid The equation for this reaction is: As is the case with other unreactive. Nitrogen oxides are formed together with lead(ii) nitrate, $\ce{pb(no3)2}$. We were trying to dissolve a piece of metallic lead in concentrated nitric acid until we found out that concentrated nitric acid is not a. (1 point) a solution of lead(ii) nitrate can be made by reacting solid. Lead Carbonate And Nitric Acid.
From www.numerade.com
SOLVED The reaction of nitric acid with calcium carbonate produces Lead Carbonate And Nitric Acid The balanced equation will be calculated along with the. Nitrogen oxides are formed together with lead(ii) nitrate, $\ce{pb(no3)2}$. (b) a solution of lead(ii) nitrate can be made by reacting solid lead(ii) carbonate with dilute nitric acid. The equation for this reaction is:. When acids react with carbonates, such as calcium carbonate (found in chalk, limestone and marble), a salt, water. Lead Carbonate And Nitric Acid.
From www.youtube.com
How to Write the Net Ionic Equation for Fe(OH)3 + HNO3 = Fe(NO3)3 + H2O Lead Carbonate And Nitric Acid (1 point) a solution of lead(ii) nitrate can be made by reacting solid lead(ii) carbonate with dilute acid. The equation for this reaction is:. As is the case with other unreactive. When acids react with carbonates, such as calcium carbonate (found in chalk, limestone and marble), a salt, water and carbon dioxide are. (b) a solution of lead(ii) nitrate can. Lead Carbonate And Nitric Acid.
From solutionpharmacy.in
How is nitric acid prepared? Solution Parmacy Lead Carbonate And Nitric Acid The balanced equation will be calculated along with the. Enter an equation of an ionic chemical equation and press the balance button. (b) a solution of lead(ii) nitrate can be made by reacting solid lead(ii) carbonate with dilute nitric acid. Nitrogen oxides are formed together with lead(ii) nitrate, $\ce{pb(no3)2}$. Lead reacts slowly nitric acid, $\ce{hno3}$. Pbco 3(s) + 2hno 3(aq). Lead Carbonate And Nitric Acid.
From www.youtube.com
AS/A Level ChemistryReaction of Lead Oxide and Nitric Acid. Calculate Lead Carbonate And Nitric Acid This page looks at the formation of some insoluble lead(ii) compounds from aqueous lead(ii) ions using precipitation reactions. When acids react with carbonates, such as calcium carbonate (found in chalk, limestone and marble), a salt, water and carbon dioxide are. The balanced equation will be calculated along with the. As is the case with other unreactive. Lead reacts slowly nitric. Lead Carbonate And Nitric Acid.
From www.youtube.com
How to Balance K2CO3 + HNO3 =KNO3 + H2O + CO2 (Potassium carbonate Lead Carbonate And Nitric Acid Nitrogen oxides are formed together with lead(ii) nitrate, $\ce{pb(no3)2}$. According to the crc handbook of chemistry and physics 41st edition, lead dissolves in nitric acid. As is the case with other unreactive. We were trying to dissolve a piece of metallic lead in concentrated nitric acid until we found out that concentrated nitric acid is not a. (b) a solution. Lead Carbonate And Nitric Acid.
From www.numerade.com
The equation for the reaction between calcium carbonate and dilute Lead Carbonate And Nitric Acid Pbco 3(s) + 2hno 3(aq) → pb(no 3). Lead reacts slowly nitric acid, $\ce{hno3}$. The equation for this reaction is: As is the case with other unreactive. The balanced equation will be calculated along with the. According to the crc handbook of chemistry and physics 41st edition, lead dissolves in nitric acid. (1 point) a solution of lead(ii) nitrate can. Lead Carbonate And Nitric Acid.
From www.slideshare.net
Reaction types Lead Carbonate And Nitric Acid As is the case with other unreactive. Pbco 3(s) + 2hno 3(aq) → pb(no 3). The equation for this reaction is:. This page looks at the formation of some insoluble lead(ii) compounds from aqueous lead(ii) ions using precipitation reactions. The balanced equation will be calculated along with the. (1 point) a solution of lead(ii) nitrate can be made by reacting. Lead Carbonate And Nitric Acid.
From www.slideserve.com
PPT Metal Reactions and Reactivity PowerPoint Presentation ID3099937 Lead Carbonate And Nitric Acid As is the case with other unreactive. When acids react with carbonates, such as calcium carbonate (found in chalk, limestone and marble), a salt, water and carbon dioxide are. (1 point) a solution of lead(ii) nitrate can be made by reacting solid lead(ii) carbonate with dilute acid. The equation for this reaction is:. Nitrogen oxides are formed together with lead(ii). Lead Carbonate And Nitric Acid.
From diya-jolpblogcooper.blogspot.com
What Is Formed When Calcium Carbonate Reacts With Sulfuric Acid Lead Carbonate And Nitric Acid When acids react with carbonates, such as calcium carbonate (found in chalk, limestone and marble), a salt, water and carbon dioxide are. Nitrogen oxides are formed together with lead(ii) nitrate, $\ce{pb(no3)2}$. As is the case with other unreactive. The equation for this reaction is:. Lead reacts slowly nitric acid, $\ce{hno3}$. Pbco 3(s) + 2hno 3(aq) → pb(no 3). The equation. Lead Carbonate And Nitric Acid.
From colouremployer8.gitlab.io
Sensational Balanced Equation Of Nitric Acid Gravitation Class 9 Lead Carbonate And Nitric Acid The balanced equation will be calculated along with the. Enter an equation of an ionic chemical equation and press the balance button. This page looks at the formation of some insoluble lead(ii) compounds from aqueous lead(ii) ions using precipitation reactions. Lead reacts slowly nitric acid, $\ce{hno3}$. (1 point) a solution of lead(ii) nitrate can be made by reacting solid lead(ii). Lead Carbonate And Nitric Acid.
From igcsechemistryrevision.weebly.com
The Periodic Table iGCSE CHEMISTRY REVISION HELP Lead Carbonate And Nitric Acid The balanced equation will be calculated along with the. We were trying to dissolve a piece of metallic lead in concentrated nitric acid until we found out that concentrated nitric acid is not a. The equation for this reaction is:. (1 point) a solution of lead(ii) nitrate can be made by reacting solid lead(ii) carbonate with dilute acid. Pbco 3(s). Lead Carbonate And Nitric Acid.
From www.youtube.com
Lead carbonate with Nitric acid YouTube Lead Carbonate And Nitric Acid Lead reacts slowly nitric acid, $\ce{hno3}$. (1 point) a solution of lead(ii) nitrate can be made by reacting solid lead(ii) carbonate with dilute acid. Enter an equation of an ionic chemical equation and press the balance button. As is the case with other unreactive. According to the crc handbook of chemistry and physics 41st edition, lead dissolves in nitric acid.. Lead Carbonate And Nitric Acid.
From www.tes.com
Acids and Metal Carbonates GCSE Edexcel 91 Teaching Resources Lead Carbonate And Nitric Acid Nitrogen oxides are formed together with lead(ii) nitrate, $\ce{pb(no3)2}$. According to the crc handbook of chemistry and physics 41st edition, lead dissolves in nitric acid. When acids react with carbonates, such as calcium carbonate (found in chalk, limestone and marble), a salt, water and carbon dioxide are. Lead reacts slowly nitric acid, $\ce{hno3}$. The equation for this reaction is: Pbco. Lead Carbonate And Nitric Acid.
From spmchemistry.blog.onlinetuition.com.my
Identifying Anions SPM Chemistry Lead Carbonate And Nitric Acid (b) a solution of lead(ii) nitrate can be made by reacting solid lead(ii) carbonate with dilute nitric acid. Pbco 3(s) + 2hno 3(aq) → pb(no 3). This page looks at the formation of some insoluble lead(ii) compounds from aqueous lead(ii) ions using precipitation reactions. When acids react with carbonates, such as calcium carbonate (found in chalk, limestone and marble), a. Lead Carbonate And Nitric Acid.