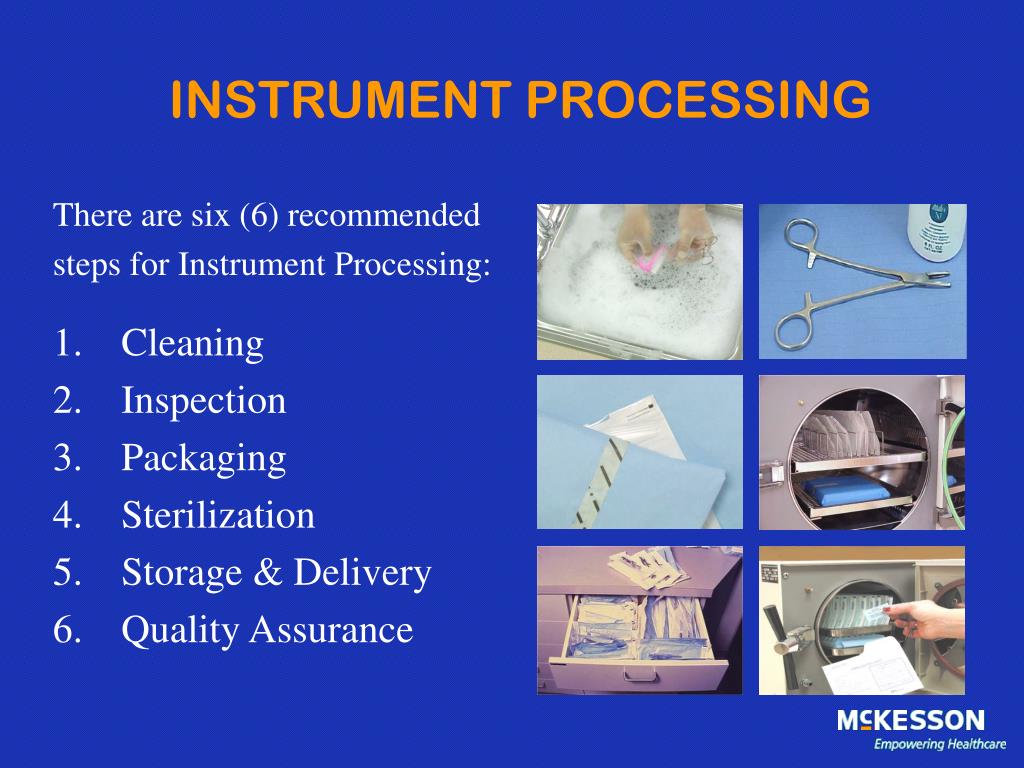
Cleaning And Storage Requirements For Sterilisation Equipment . reprocessing is a multistep process that includes cleaning, inspection and assembly, functional testing (if applicable),. Rotate stock so that items sterilised earlier are used. Only handle packs with clean hands. cleaning and sterilising reusable equipment and instruments. All reusable equipment and instruments that are used in a skin penetration procedure. reprocessing of reusable equipment and devices meets current best practice and is consistent with recurrent national. a released rmd/other device shall be recalled in a timely manner where there is evidence of failure during the. where visiting health professionals supply their own sterile equipment or sterile loan equipment is used, organisations are. when cool, store sterilised packs in clean enclosed storage area.
from www.slideserve.com
when cool, store sterilised packs in clean enclosed storage area. Rotate stock so that items sterilised earlier are used. Only handle packs with clean hands. reprocessing is a multistep process that includes cleaning, inspection and assembly, functional testing (if applicable),. All reusable equipment and instruments that are used in a skin penetration procedure. a released rmd/other device shall be recalled in a timely manner where there is evidence of failure during the. cleaning and sterilising reusable equipment and instruments. reprocessing of reusable equipment and devices meets current best practice and is consistent with recurrent national. where visiting health professionals supply their own sterile equipment or sterile loan equipment is used, organisations are.
PPT Cleaning, Packaging and Sterilization of Instruments PowerPoint
Cleaning And Storage Requirements For Sterilisation Equipment Rotate stock so that items sterilised earlier are used. Only handle packs with clean hands. when cool, store sterilised packs in clean enclosed storage area. cleaning and sterilising reusable equipment and instruments. reprocessing of reusable equipment and devices meets current best practice and is consistent with recurrent national. All reusable equipment and instruments that are used in a skin penetration procedure. where visiting health professionals supply their own sterile equipment or sterile loan equipment is used, organisations are. reprocessing is a multistep process that includes cleaning, inspection and assembly, functional testing (if applicable),. Rotate stock so that items sterilised earlier are used. a released rmd/other device shall be recalled in a timely manner where there is evidence of failure during the.
From www.vet-way.com
Cleaning And Sterilisation Best Practices For Surgical Equipment Vet Cleaning And Storage Requirements For Sterilisation Equipment a released rmd/other device shall be recalled in a timely manner where there is evidence of failure during the. All reusable equipment and instruments that are used in a skin penetration procedure. Only handle packs with clean hands. when cool, store sterilised packs in clean enclosed storage area. Rotate stock so that items sterilised earlier are used. . Cleaning And Storage Requirements For Sterilisation Equipment.
From www.slideserve.com
PPT Cleaning, Packaging and Sterilization of Instruments PowerPoint Cleaning And Storage Requirements For Sterilisation Equipment All reusable equipment and instruments that are used in a skin penetration procedure. Only handle packs with clean hands. a released rmd/other device shall be recalled in a timely manner where there is evidence of failure during the. where visiting health professionals supply their own sterile equipment or sterile loan equipment is used, organisations are. Rotate stock so. Cleaning And Storage Requirements For Sterilisation Equipment.
From www.slideserve.com
PPT Cleaning, Packaging and Sterilization of Instruments PowerPoint Cleaning And Storage Requirements For Sterilisation Equipment reprocessing is a multistep process that includes cleaning, inspection and assembly, functional testing (if applicable),. where visiting health professionals supply their own sterile equipment or sterile loan equipment is used, organisations are. All reusable equipment and instruments that are used in a skin penetration procedure. a released rmd/other device shall be recalled in a timely manner where. Cleaning And Storage Requirements For Sterilisation Equipment.
From www.cleanroom-industries.com
Cleaning & Sterilization Cleanroom Cleaning And Storage Requirements For Sterilisation Equipment cleaning and sterilising reusable equipment and instruments. Only handle packs with clean hands. where visiting health professionals supply their own sterile equipment or sterile loan equipment is used, organisations are. a released rmd/other device shall be recalled in a timely manner where there is evidence of failure during the. Rotate stock so that items sterilised earlier are. Cleaning And Storage Requirements For Sterilisation Equipment.
From www.halton.com
Sterilisation room solution Halton Cleaning And Storage Requirements For Sterilisation Equipment reprocessing is a multistep process that includes cleaning, inspection and assembly, functional testing (if applicable),. Only handle packs with clean hands. Rotate stock so that items sterilised earlier are used. when cool, store sterilised packs in clean enclosed storage area. where visiting health professionals supply their own sterile equipment or sterile loan equipment is used, organisations are.. Cleaning And Storage Requirements For Sterilisation Equipment.
From www.universalcompanies.com
Single UV Sterilizer, 120V Cleaning And Storage Requirements For Sterilisation Equipment where visiting health professionals supply their own sterile equipment or sterile loan equipment is used, organisations are. reprocessing is a multistep process that includes cleaning, inspection and assembly, functional testing (if applicable),. Only handle packs with clean hands. a released rmd/other device shall be recalled in a timely manner where there is evidence of failure during the.. Cleaning And Storage Requirements For Sterilisation Equipment.
From www.youtube.com
Guidance for Cleaning, Disinfection and Sterilization of Reusable Cleaning And Storage Requirements For Sterilisation Equipment reprocessing is a multistep process that includes cleaning, inspection and assembly, functional testing (if applicable),. Rotate stock so that items sterilised earlier are used. a released rmd/other device shall be recalled in a timely manner where there is evidence of failure during the. All reusable equipment and instruments that are used in a skin penetration procedure. where. Cleaning And Storage Requirements For Sterilisation Equipment.
From cssdtechnicianhub.com
Sterile Processing Guidelines and Objectives CSSD Technician Hub Cleaning And Storage Requirements For Sterilisation Equipment reprocessing is a multistep process that includes cleaning, inspection and assembly, functional testing (if applicable),. when cool, store sterilised packs in clean enclosed storage area. All reusable equipment and instruments that are used in a skin penetration procedure. a released rmd/other device shall be recalled in a timely manner where there is evidence of failure during the.. Cleaning And Storage Requirements For Sterilisation Equipment.
From microbeonline.com
Sterilization and Disinfection Methods Microbe Online Cleaning And Storage Requirements For Sterilisation Equipment when cool, store sterilised packs in clean enclosed storage area. reprocessing is a multistep process that includes cleaning, inspection and assembly, functional testing (if applicable),. Rotate stock so that items sterilised earlier are used. cleaning and sterilising reusable equipment and instruments. All reusable equipment and instruments that are used in a skin penetration procedure. a released. Cleaning And Storage Requirements For Sterilisation Equipment.
From www.yierhealth.com
The Importance of Proper Cleaning and Sterilisation of Anaesthetic Cleaning And Storage Requirements For Sterilisation Equipment Only handle packs with clean hands. where visiting health professionals supply their own sterile equipment or sterile loan equipment is used, organisations are. Rotate stock so that items sterilised earlier are used. when cool, store sterilised packs in clean enclosed storage area. cleaning and sterilising reusable equipment and instruments. reprocessing of reusable equipment and devices meets. Cleaning And Storage Requirements For Sterilisation Equipment.
From medicom-asia.com
Sterilization Products Asia Cleaning And Storage Requirements For Sterilisation Equipment cleaning and sterilising reusable equipment and instruments. when cool, store sterilised packs in clean enclosed storage area. where visiting health professionals supply their own sterile equipment or sterile loan equipment is used, organisations are. All reusable equipment and instruments that are used in a skin penetration procedure. Rotate stock so that items sterilised earlier are used. . Cleaning And Storage Requirements For Sterilisation Equipment.
From www.geomedsdvo.com
Sterile Processing Supplies GeoMed Sterilization Medical Equipment Cleaning And Storage Requirements For Sterilisation Equipment when cool, store sterilised packs in clean enclosed storage area. Only handle packs with clean hands. Rotate stock so that items sterilised earlier are used. where visiting health professionals supply their own sterile equipment or sterile loan equipment is used, organisations are. cleaning and sterilising reusable equipment and instruments. reprocessing is a multistep process that includes. Cleaning And Storage Requirements For Sterilisation Equipment.
From dxoynfrei.blob.core.windows.net
Proper Sterilization In An Autoclave at Donald Lamb blog Cleaning And Storage Requirements For Sterilisation Equipment Rotate stock so that items sterilised earlier are used. Only handle packs with clean hands. when cool, store sterilised packs in clean enclosed storage area. reprocessing of reusable equipment and devices meets current best practice and is consistent with recurrent national. reprocessing is a multistep process that includes cleaning, inspection and assembly, functional testing (if applicable),. All. Cleaning And Storage Requirements For Sterilisation Equipment.
From www.medicaldesignandoutsourcing.com
A look at the industrial sterilization market Medical Design and Cleaning And Storage Requirements For Sterilisation Equipment reprocessing of reusable equipment and devices meets current best practice and is consistent with recurrent national. a released rmd/other device shall be recalled in a timely manner where there is evidence of failure during the. when cool, store sterilised packs in clean enclosed storage area. Only handle packs with clean hands. Rotate stock so that items sterilised. Cleaning And Storage Requirements For Sterilisation Equipment.
From www.scribd.com
Guidelines For Cleaning Disinfection and Sterilisation of Patient Care Cleaning And Storage Requirements For Sterilisation Equipment where visiting health professionals supply their own sterile equipment or sterile loan equipment is used, organisations are. when cool, store sterilised packs in clean enclosed storage area. reprocessing of reusable equipment and devices meets current best practice and is consistent with recurrent national. cleaning and sterilising reusable equipment and instruments. reprocessing is a multistep process. Cleaning And Storage Requirements For Sterilisation Equipment.
From www.iccs-home.com
Temperature and Humidity Requirements for Sterile Supplies — Infection Cleaning And Storage Requirements For Sterilisation Equipment Only handle packs with clean hands. Rotate stock so that items sterilised earlier are used. where visiting health professionals supply their own sterile equipment or sterile loan equipment is used, organisations are. All reusable equipment and instruments that are used in a skin penetration procedure. cleaning and sterilising reusable equipment and instruments. reprocessing is a multistep process. Cleaning And Storage Requirements For Sterilisation Equipment.
From www.scribd.com
Guidelines for Ensuring Proper Cleaning, Disinfection, and Cleaning And Storage Requirements For Sterilisation Equipment cleaning and sterilising reusable equipment and instruments. when cool, store sterilised packs in clean enclosed storage area. a released rmd/other device shall be recalled in a timely manner where there is evidence of failure during the. reprocessing is a multistep process that includes cleaning, inspection and assembly, functional testing (if applicable),. reprocessing of reusable equipment. Cleaning And Storage Requirements For Sterilisation Equipment.
From www.health.nsw.gov.au
Cleaning and sterilising reusable equipment and instruments Fact sheets Cleaning And Storage Requirements For Sterilisation Equipment a released rmd/other device shall be recalled in a timely manner where there is evidence of failure during the. Only handle packs with clean hands. cleaning and sterilising reusable equipment and instruments. reprocessing of reusable equipment and devices meets current best practice and is consistent with recurrent national. when cool, store sterilised packs in clean enclosed. Cleaning And Storage Requirements For Sterilisation Equipment.
From yourdenture.com
Sterilization and Disinfection (asepsis control) Brookswood Denture Cleaning And Storage Requirements For Sterilisation Equipment cleaning and sterilising reusable equipment and instruments. All reusable equipment and instruments that are used in a skin penetration procedure. reprocessing of reusable equipment and devices meets current best practice and is consistent with recurrent national. Only handle packs with clean hands. a released rmd/other device shall be recalled in a timely manner where there is evidence. Cleaning And Storage Requirements For Sterilisation Equipment.
From dxogxlgae.blob.core.windows.net
Autoclave Technology at Diana McColl blog Cleaning And Storage Requirements For Sterilisation Equipment Rotate stock so that items sterilised earlier are used. Only handle packs with clean hands. reprocessing is a multistep process that includes cleaning, inspection and assembly, functional testing (if applicable),. All reusable equipment and instruments that are used in a skin penetration procedure. reprocessing of reusable equipment and devices meets current best practice and is consistent with recurrent. Cleaning And Storage Requirements For Sterilisation Equipment.
From microbeonline.com
Autoclave Sterilization Principle, Procedure, Types, Uses • Microbe Online Cleaning And Storage Requirements For Sterilisation Equipment reprocessing of reusable equipment and devices meets current best practice and is consistent with recurrent national. Rotate stock so that items sterilised earlier are used. All reusable equipment and instruments that are used in a skin penetration procedure. where visiting health professionals supply their own sterile equipment or sterile loan equipment is used, organisations are. Only handle packs. Cleaning And Storage Requirements For Sterilisation Equipment.
From studylib.net
Cleaning, Disinfection, and Sterilisation Cleaning And Storage Requirements For Sterilisation Equipment All reusable equipment and instruments that are used in a skin penetration procedure. Rotate stock so that items sterilised earlier are used. a released rmd/other device shall be recalled in a timely manner where there is evidence of failure during the. reprocessing of reusable equipment and devices meets current best practice and is consistent with recurrent national. . Cleaning And Storage Requirements For Sterilisation Equipment.
From ndimedical.eu
Sterilisation and desinfection guide NDI Medical Cleaning And Storage Requirements For Sterilisation Equipment cleaning and sterilising reusable equipment and instruments. reprocessing of reusable equipment and devices meets current best practice and is consistent with recurrent national. Only handle packs with clean hands. reprocessing is a multistep process that includes cleaning, inspection and assembly, functional testing (if applicable),. All reusable equipment and instruments that are used in a skin penetration procedure.. Cleaning And Storage Requirements For Sterilisation Equipment.
From www.alamy.com
Surgical instruments cleaning, sterilization plant. Hospital Stock Cleaning And Storage Requirements For Sterilisation Equipment reprocessing of reusable equipment and devices meets current best practice and is consistent with recurrent national. Rotate stock so that items sterilised earlier are used. a released rmd/other device shall be recalled in a timely manner where there is evidence of failure during the. where visiting health professionals supply their own sterile equipment or sterile loan equipment. Cleaning And Storage Requirements For Sterilisation Equipment.
From www.sepsservices.com
5 Common Methods of Lab Sterilization SEPS Services Cleaning And Storage Requirements For Sterilisation Equipment a released rmd/other device shall be recalled in a timely manner where there is evidence of failure during the. where visiting health professionals supply their own sterile equipment or sterile loan equipment is used, organisations are. All reusable equipment and instruments that are used in a skin penetration procedure. cleaning and sterilising reusable equipment and instruments. . Cleaning And Storage Requirements For Sterilisation Equipment.
From www.dsidirect.com
The Importance of a Clean and Sterile Supply Storage Process in a Cleaning And Storage Requirements For Sterilisation Equipment Only handle packs with clean hands. All reusable equipment and instruments that are used in a skin penetration procedure. when cool, store sterilised packs in clean enclosed storage area. where visiting health professionals supply their own sterile equipment or sterile loan equipment is used, organisations are. a released rmd/other device shall be recalled in a timely manner. Cleaning And Storage Requirements For Sterilisation Equipment.
From www.vrogue.co
Proper Sterilization Of Surgical Instruments Youtube vrogue.co Cleaning And Storage Requirements For Sterilisation Equipment cleaning and sterilising reusable equipment and instruments. reprocessing of reusable equipment and devices meets current best practice and is consistent with recurrent national. All reusable equipment and instruments that are used in a skin penetration procedure. a released rmd/other device shall be recalled in a timely manner where there is evidence of failure during the. Only handle. Cleaning And Storage Requirements For Sterilisation Equipment.
From www.ahpmedicals.com
How to Sterilize Medical Equipment Cleaning, Disinfection, and Cleaning And Storage Requirements For Sterilisation Equipment All reusable equipment and instruments that are used in a skin penetration procedure. a released rmd/other device shall be recalled in a timely manner where there is evidence of failure during the. reprocessing is a multistep process that includes cleaning, inspection and assembly, functional testing (if applicable),. Rotate stock so that items sterilised earlier are used. where. Cleaning And Storage Requirements For Sterilisation Equipment.
From www.casemed.com
"Managing Sterile Processing" Cleaning And Storage Requirements For Sterilisation Equipment when cool, store sterilised packs in clean enclosed storage area. Only handle packs with clean hands. cleaning and sterilising reusable equipment and instruments. Rotate stock so that items sterilised earlier are used. a released rmd/other device shall be recalled in a timely manner where there is evidence of failure during the. reprocessing of reusable equipment and. Cleaning And Storage Requirements For Sterilisation Equipment.
From www.careers.govt.nz
Sterilising Technician Cleaning And Storage Requirements For Sterilisation Equipment when cool, store sterilised packs in clean enclosed storage area. All reusable equipment and instruments that are used in a skin penetration procedure. where visiting health professionals supply their own sterile equipment or sterile loan equipment is used, organisations are. Rotate stock so that items sterilised earlier are used. Only handle packs with clean hands. reprocessing of. Cleaning And Storage Requirements For Sterilisation Equipment.
From oxymedz.com
Sterilization Of Medical Equipment OxyMed Cleaning And Storage Requirements For Sterilisation Equipment reprocessing is a multistep process that includes cleaning, inspection and assembly, functional testing (if applicable),. cleaning and sterilising reusable equipment and instruments. Only handle packs with clean hands. when cool, store sterilised packs in clean enclosed storage area. All reusable equipment and instruments that are used in a skin penetration procedure. a released rmd/other device shall. Cleaning And Storage Requirements For Sterilisation Equipment.
From dxokaikxl.blob.core.windows.net
Sterilization Methods Medical Device at Dave Burdick blog Cleaning And Storage Requirements For Sterilisation Equipment Only handle packs with clean hands. where visiting health professionals supply their own sterile equipment or sterile loan equipment is used, organisations are. All reusable equipment and instruments that are used in a skin penetration procedure. cleaning and sterilising reusable equipment and instruments. when cool, store sterilised packs in clean enclosed storage area. reprocessing of reusable. Cleaning And Storage Requirements For Sterilisation Equipment.
From dxoskqclm.blob.core.windows.net
When Using An Autoclave To Sterilize Instruments You Must at Ronnie Cleaning And Storage Requirements For Sterilisation Equipment a released rmd/other device shall be recalled in a timely manner where there is evidence of failure during the. Rotate stock so that items sterilised earlier are used. reprocessing of reusable equipment and devices meets current best practice and is consistent with recurrent national. when cool, store sterilised packs in clean enclosed storage area. reprocessing is. Cleaning And Storage Requirements For Sterilisation Equipment.
From www.healthcare-spaces.com
Ensuring compliance with the new sterilisation standards Healthcare Cleaning And Storage Requirements For Sterilisation Equipment cleaning and sterilising reusable equipment and instruments. Rotate stock so that items sterilised earlier are used. reprocessing of reusable equipment and devices meets current best practice and is consistent with recurrent national. where visiting health professionals supply their own sterile equipment or sterile loan equipment is used, organisations are. when cool, store sterilised packs in clean. Cleaning And Storage Requirements For Sterilisation Equipment.
From cssdtechnicianhub.com
Central Sterile Processing and Distribution CSSD Technician Hub Cleaning And Storage Requirements For Sterilisation Equipment Rotate stock so that items sterilised earlier are used. reprocessing of reusable equipment and devices meets current best practice and is consistent with recurrent national. cleaning and sterilising reusable equipment and instruments. Only handle packs with clean hands. when cool, store sterilised packs in clean enclosed storage area. reprocessing is a multistep process that includes cleaning,. Cleaning And Storage Requirements For Sterilisation Equipment.