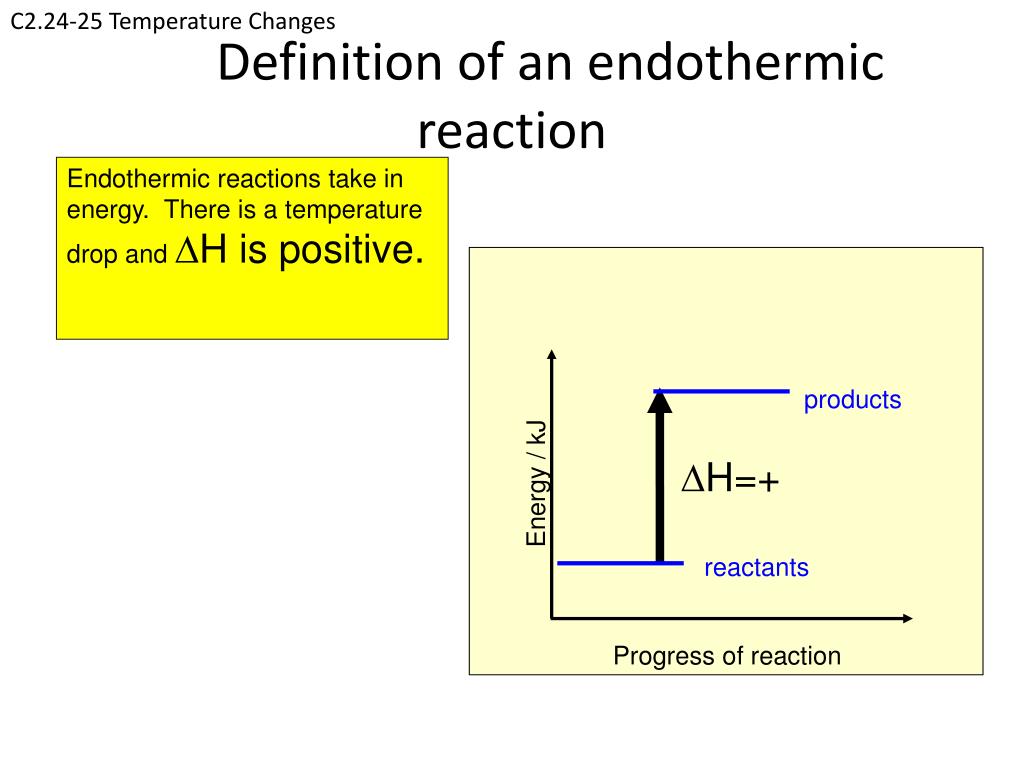
Endothermic Non Examples . Explore the definition, characteristics, examples, equations, and energy level diagrams of. some examples of endothermic reactions are: green plants are capable of synthesizing glucose (c 6 h 12 o 6) from carbon dioxide (co 2) and water (h 2 o) by. examples of endothermic reactions include photosynthesis, dissolving salt in water, and chemical cold packs. an example of an easy endothermic reaction is dissolving potassium chloride (sold as a salt substitute) in your. learn the process of endothermic reactions. The reaction between ethanoic acid and sodium carbonate. The formation of barium thiocyanate from ammonium thiocyanate and barium hydroxide is so endothermic.
from www.slideserve.com
some examples of endothermic reactions are: learn the process of endothermic reactions. The reaction between ethanoic acid and sodium carbonate. examples of endothermic reactions include photosynthesis, dissolving salt in water, and chemical cold packs. The formation of barium thiocyanate from ammonium thiocyanate and barium hydroxide is so endothermic. Explore the definition, characteristics, examples, equations, and energy level diagrams of. green plants are capable of synthesizing glucose (c 6 h 12 o 6) from carbon dioxide (co 2) and water (h 2 o) by. an example of an easy endothermic reaction is dissolving potassium chloride (sold as a salt substitute) in your.
PPT Topic 5 Chemical Reactions Revision Checklist PowerPoint
Endothermic Non Examples some examples of endothermic reactions are: Explore the definition, characteristics, examples, equations, and energy level diagrams of. an example of an easy endothermic reaction is dissolving potassium chloride (sold as a salt substitute) in your. some examples of endothermic reactions are: The reaction between ethanoic acid and sodium carbonate. green plants are capable of synthesizing glucose (c 6 h 12 o 6) from carbon dioxide (co 2) and water (h 2 o) by. The formation of barium thiocyanate from ammonium thiocyanate and barium hydroxide is so endothermic. learn the process of endothermic reactions. examples of endothermic reactions include photosynthesis, dissolving salt in water, and chemical cold packs.
From www.slideserve.com
PPT Topic 5 Chemical Reactions Revision Checklist PowerPoint Endothermic Non Examples examples of endothermic reactions include photosynthesis, dissolving salt in water, and chemical cold packs. The formation of barium thiocyanate from ammonium thiocyanate and barium hydroxide is so endothermic. some examples of endothermic reactions are: learn the process of endothermic reactions. an example of an easy endothermic reaction is dissolving potassium chloride (sold as a salt substitute). Endothermic Non Examples.
From www.yaclass.in
Exothermic and Endothermic chemical changes — lesson. Science State Endothermic Non Examples an example of an easy endothermic reaction is dissolving potassium chloride (sold as a salt substitute) in your. examples of endothermic reactions include photosynthesis, dissolving salt in water, and chemical cold packs. learn the process of endothermic reactions. The reaction between ethanoic acid and sodium carbonate. green plants are capable of synthesizing glucose (c 6 h. Endothermic Non Examples.
From www.difference101.com
Endothermic vs. Exothermic 5 Key Differences, Pros & Cons, Examples Endothermic Non Examples learn the process of endothermic reactions. green plants are capable of synthesizing glucose (c 6 h 12 o 6) from carbon dioxide (co 2) and water (h 2 o) by. The reaction between ethanoic acid and sodium carbonate. some examples of endothermic reactions are: examples of endothermic reactions include photosynthesis, dissolving salt in water, and chemical. Endothermic Non Examples.
From eduinput.com
Difference Between Endothermic And Exothermic Reactions Endothermic Non Examples some examples of endothermic reactions are: green plants are capable of synthesizing glucose (c 6 h 12 o 6) from carbon dioxide (co 2) and water (h 2 o) by. an example of an easy endothermic reaction is dissolving potassium chloride (sold as a salt substitute) in your. Explore the definition, characteristics, examples, equations, and energy level. Endothermic Non Examples.
From diagramlibpapudocoe.z13.web.core.windows.net
Energy Diagram Chemistry Explained Endothermic Non Examples The reaction between ethanoic acid and sodium carbonate. Explore the definition, characteristics, examples, equations, and energy level diagrams of. green plants are capable of synthesizing glucose (c 6 h 12 o 6) from carbon dioxide (co 2) and water (h 2 o) by. some examples of endothermic reactions are: an example of an easy endothermic reaction is. Endothermic Non Examples.
From byjus.com
Difference Between Endothermic and Exothermic Reactions Chemistry Endothermic Non Examples Explore the definition, characteristics, examples, equations, and energy level diagrams of. an example of an easy endothermic reaction is dissolving potassium chloride (sold as a salt substitute) in your. examples of endothermic reactions include photosynthesis, dissolving salt in water, and chemical cold packs. some examples of endothermic reactions are: learn the process of endothermic reactions. . Endothermic Non Examples.
From www.slideserve.com
PPT Phase Changes PowerPoint Presentation, free download ID2202556 Endothermic Non Examples an example of an easy endothermic reaction is dissolving potassium chloride (sold as a salt substitute) in your. Explore the definition, characteristics, examples, equations, and energy level diagrams of. The formation of barium thiocyanate from ammonium thiocyanate and barium hydroxide is so endothermic. examples of endothermic reactions include photosynthesis, dissolving salt in water, and chemical cold packs. . Endothermic Non Examples.
From mavink.com
Endothermic And Exothermic Reactions Examples Endothermic Non Examples learn the process of endothermic reactions. some examples of endothermic reactions are: an example of an easy endothermic reaction is dissolving potassium chloride (sold as a salt substitute) in your. green plants are capable of synthesizing glucose (c 6 h 12 o 6) from carbon dioxide (co 2) and water (h 2 o) by. examples. Endothermic Non Examples.
From quizizz.com
8 Endothemic Vs Exothermic Reactions Science Quizizz Endothermic Non Examples examples of endothermic reactions include photosynthesis, dissolving salt in water, and chemical cold packs. some examples of endothermic reactions are: Explore the definition, characteristics, examples, equations, and energy level diagrams of. learn the process of endothermic reactions. The reaction between ethanoic acid and sodium carbonate. The formation of barium thiocyanate from ammonium thiocyanate and barium hydroxide is. Endothermic Non Examples.
From www.vecteezy.com
Enthalpy Endothermic versus Endothermic biochemistry vector Endothermic Non Examples some examples of endothermic reactions are: an example of an easy endothermic reaction is dissolving potassium chloride (sold as a salt substitute) in your. The reaction between ethanoic acid and sodium carbonate. learn the process of endothermic reactions. green plants are capable of synthesizing glucose (c 6 h 12 o 6) from carbon dioxide (co 2). Endothermic Non Examples.
From khatiar1955a.altervista.org
The Difference Between Endothermic and Exothermic Reactions Endothermic Non Examples The formation of barium thiocyanate from ammonium thiocyanate and barium hydroxide is so endothermic. learn the process of endothermic reactions. examples of endothermic reactions include photosynthesis, dissolving salt in water, and chemical cold packs. Explore the definition, characteristics, examples, equations, and energy level diagrams of. some examples of endothermic reactions are: The reaction between ethanoic acid and. Endothermic Non Examples.
From wiredatatisse56wr.z22.web.core.windows.net
Endothermic Reaction Energy Profile Diagram Endothermic Non Examples Explore the definition, characteristics, examples, equations, and energy level diagrams of. green plants are capable of synthesizing glucose (c 6 h 12 o 6) from carbon dioxide (co 2) and water (h 2 o) by. some examples of endothermic reactions are: an example of an easy endothermic reaction is dissolving potassium chloride (sold as a salt substitute). Endothermic Non Examples.
From 5differencebetween.com
5 Difference Between Endothermic and Exothermic Reaction Endothermic Endothermic Non Examples learn the process of endothermic reactions. green plants are capable of synthesizing glucose (c 6 h 12 o 6) from carbon dioxide (co 2) and water (h 2 o) by. Explore the definition, characteristics, examples, equations, and energy level diagrams of. an example of an easy endothermic reaction is dissolving potassium chloride (sold as a salt substitute). Endothermic Non Examples.
From eduinput.com
Endothermic ReactionsCharacteristics, Identification, and Examples Endothermic Non Examples green plants are capable of synthesizing glucose (c 6 h 12 o 6) from carbon dioxide (co 2) and water (h 2 o) by. Explore the definition, characteristics, examples, equations, and energy level diagrams of. The formation of barium thiocyanate from ammonium thiocyanate and barium hydroxide is so endothermic. examples of endothermic reactions include photosynthesis, dissolving salt in. Endothermic Non Examples.
From www.diffzy.com
Exothermic vs. Endothermic Reactions What's the Difference (With Table) Endothermic Non Examples Explore the definition, characteristics, examples, equations, and energy level diagrams of. some examples of endothermic reactions are: an example of an easy endothermic reaction is dissolving potassium chloride (sold as a salt substitute) in your. The reaction between ethanoic acid and sodium carbonate. examples of endothermic reactions include photosynthesis, dissolving salt in water, and chemical cold packs.. Endothermic Non Examples.
From pediaa.com
Difference Between Endothermic and Exothermic Reactions Definition Endothermic Non Examples examples of endothermic reactions include photosynthesis, dissolving salt in water, and chemical cold packs. The formation of barium thiocyanate from ammonium thiocyanate and barium hydroxide is so endothermic. The reaction between ethanoic acid and sodium carbonate. an example of an easy endothermic reaction is dissolving potassium chloride (sold as a salt substitute) in your. some examples of. Endothermic Non Examples.
From 7esl.com
Exothermic vs. Endothermic The Main Difference • 7ESL Endothermic Non Examples some examples of endothermic reactions are: The reaction between ethanoic acid and sodium carbonate. Explore the definition, characteristics, examples, equations, and energy level diagrams of. The formation of barium thiocyanate from ammonium thiocyanate and barium hydroxide is so endothermic. green plants are capable of synthesizing glucose (c 6 h 12 o 6) from carbon dioxide (co 2) and. Endothermic Non Examples.
From www.slideserve.com
PPT Endothermic and Exothermic Reactions PowerPoint Presentation ID Endothermic Non Examples Explore the definition, characteristics, examples, equations, and energy level diagrams of. an example of an easy endothermic reaction is dissolving potassium chloride (sold as a salt substitute) in your. green plants are capable of synthesizing glucose (c 6 h 12 o 6) from carbon dioxide (co 2) and water (h 2 o) by. some examples of endothermic. Endothermic Non Examples.
From 7esl.com
Exothermic vs. Endothermic The Main Difference • 7ESL Endothermic Non Examples examples of endothermic reactions include photosynthesis, dissolving salt in water, and chemical cold packs. The formation of barium thiocyanate from ammonium thiocyanate and barium hydroxide is so endothermic. The reaction between ethanoic acid and sodium carbonate. an example of an easy endothermic reaction is dissolving potassium chloride (sold as a salt substitute) in your. Explore the definition, characteristics,. Endothermic Non Examples.
From www.worksheetsplanet.com
What is an Endothermic Reaction Definition & Example Endothermic Non Examples learn the process of endothermic reactions. The formation of barium thiocyanate from ammonium thiocyanate and barium hydroxide is so endothermic. some examples of endothermic reactions are: examples of endothermic reactions include photosynthesis, dissolving salt in water, and chemical cold packs. an example of an easy endothermic reaction is dissolving potassium chloride (sold as a salt substitute). Endothermic Non Examples.
From dxosvaiso.blob.core.windows.net
Endothermic Reaction Of Respiration at Calvin Petillo blog Endothermic Non Examples The reaction between ethanoic acid and sodium carbonate. Explore the definition, characteristics, examples, equations, and energy level diagrams of. an example of an easy endothermic reaction is dissolving potassium chloride (sold as a salt substitute) in your. The formation of barium thiocyanate from ammonium thiocyanate and barium hydroxide is so endothermic. learn the process of endothermic reactions. . Endothermic Non Examples.
From whatisdiffer.com
Difference Between Endothermic And Exothermic Reactions? Endothermic Non Examples examples of endothermic reactions include photosynthesis, dissolving salt in water, and chemical cold packs. The reaction between ethanoic acid and sodium carbonate. green plants are capable of synthesizing glucose (c 6 h 12 o 6) from carbon dioxide (co 2) and water (h 2 o) by. learn the process of endothermic reactions. some examples of endothermic. Endothermic Non Examples.
From www.difference101.com
Endothermic vs. Exothermic 5 Key Differences, Pros & Cons, Examples Endothermic Non Examples some examples of endothermic reactions are: learn the process of endothermic reactions. The formation of barium thiocyanate from ammonium thiocyanate and barium hydroxide is so endothermic. The reaction between ethanoic acid and sodium carbonate. an example of an easy endothermic reaction is dissolving potassium chloride (sold as a salt substitute) in your. Explore the definition, characteristics, examples,. Endothermic Non Examples.
From inberp.info
Differences between exothermic and endothermic reactions (2023) Endothermic Non Examples examples of endothermic reactions include photosynthesis, dissolving salt in water, and chemical cold packs. Explore the definition, characteristics, examples, equations, and energy level diagrams of. green plants are capable of synthesizing glucose (c 6 h 12 o 6) from carbon dioxide (co 2) and water (h 2 o) by. The reaction between ethanoic acid and sodium carbonate. The. Endothermic Non Examples.
From a-z-animals.com
Endothermic vs. Exothermic Reactions Key Differences and Examples A Endothermic Non Examples The formation of barium thiocyanate from ammonium thiocyanate and barium hydroxide is so endothermic. green plants are capable of synthesizing glucose (c 6 h 12 o 6) from carbon dioxide (co 2) and water (h 2 o) by. Explore the definition, characteristics, examples, equations, and energy level diagrams of. learn the process of endothermic reactions. an example. Endothermic Non Examples.
From classnotes123.com
What does one mean by exothermic and endothermic reactions? Give Endothermic Non Examples green plants are capable of synthesizing glucose (c 6 h 12 o 6) from carbon dioxide (co 2) and water (h 2 o) by. Explore the definition, characteristics, examples, equations, and energy level diagrams of. The reaction between ethanoic acid and sodium carbonate. an example of an easy endothermic reaction is dissolving potassium chloride (sold as a salt. Endothermic Non Examples.
From www.teachoo.com
Which of the reactions is an endothermic reaction? MCQ Science Endothermic Non Examples Explore the definition, characteristics, examples, equations, and energy level diagrams of. green plants are capable of synthesizing glucose (c 6 h 12 o 6) from carbon dioxide (co 2) and water (h 2 o) by. an example of an easy endothermic reaction is dissolving potassium chloride (sold as a salt substitute) in your. examples of endothermic reactions. Endothermic Non Examples.
From mmerevise.co.uk
Endothermic and Exothermic Reactions Revision MME Endothermic Non Examples an example of an easy endothermic reaction is dissolving potassium chloride (sold as a salt substitute) in your. green plants are capable of synthesizing glucose (c 6 h 12 o 6) from carbon dioxide (co 2) and water (h 2 o) by. examples of endothermic reactions include photosynthesis, dissolving salt in water, and chemical cold packs. . Endothermic Non Examples.
From online-learning-college.com
Exothermic and endothermic reactions Key differences Endothermic Non Examples Explore the definition, characteristics, examples, equations, and energy level diagrams of. an example of an easy endothermic reaction is dissolving potassium chloride (sold as a salt substitute) in your. The reaction between ethanoic acid and sodium carbonate. learn the process of endothermic reactions. examples of endothermic reactions include photosynthesis, dissolving salt in water, and chemical cold packs.. Endothermic Non Examples.
From www.slideserve.com
PPT 6.02 ChemLive Exothermic and Exothermic Reactions PowerPoint Endothermic Non Examples The reaction between ethanoic acid and sodium carbonate. The formation of barium thiocyanate from ammonium thiocyanate and barium hydroxide is so endothermic. Explore the definition, characteristics, examples, equations, and energy level diagrams of. learn the process of endothermic reactions. examples of endothermic reactions include photosynthesis, dissolving salt in water, and chemical cold packs. green plants are capable. Endothermic Non Examples.
From sciencenotes.org
Endothermic Reactions Definition and Examples Endothermic Non Examples Explore the definition, characteristics, examples, equations, and energy level diagrams of. The reaction between ethanoic acid and sodium carbonate. green plants are capable of synthesizing glucose (c 6 h 12 o 6) from carbon dioxide (co 2) and water (h 2 o) by. an example of an easy endothermic reaction is dissolving potassium chloride (sold as a salt. Endothermic Non Examples.
From www.studyorgo.com
How to Interpret Thermodynamics of Reactions Endothermic Non Examples some examples of endothermic reactions are: examples of endothermic reactions include photosynthesis, dissolving salt in water, and chemical cold packs. an example of an easy endothermic reaction is dissolving potassium chloride (sold as a salt substitute) in your. green plants are capable of synthesizing glucose (c 6 h 12 o 6) from carbon dioxide (co 2). Endothermic Non Examples.
From lessonschoolallodial.z13.web.core.windows.net
Endothermic Vs Exothermic Chemical Reactions Endothermic Non Examples The reaction between ethanoic acid and sodium carbonate. learn the process of endothermic reactions. green plants are capable of synthesizing glucose (c 6 h 12 o 6) from carbon dioxide (co 2) and water (h 2 o) by. an example of an easy endothermic reaction is dissolving potassium chloride (sold as a salt substitute) in your. The. Endothermic Non Examples.
From www.animalia-life.club
Endothermic Reaction Examples For Kids Endothermic Non Examples The reaction between ethanoic acid and sodium carbonate. Explore the definition, characteristics, examples, equations, and energy level diagrams of. green plants are capable of synthesizing glucose (c 6 h 12 o 6) from carbon dioxide (co 2) and water (h 2 o) by. examples of endothermic reactions include photosynthesis, dissolving salt in water, and chemical cold packs. . Endothermic Non Examples.
From classzonesalicetum.z14.web.core.windows.net
Identify Endothermic And Exothermic Reactions Endothermic Non Examples green plants are capable of synthesizing glucose (c 6 h 12 o 6) from carbon dioxide (co 2) and water (h 2 o) by. an example of an easy endothermic reaction is dissolving potassium chloride (sold as a salt substitute) in your. The formation of barium thiocyanate from ammonium thiocyanate and barium hydroxide is so endothermic. learn. Endothermic Non Examples.