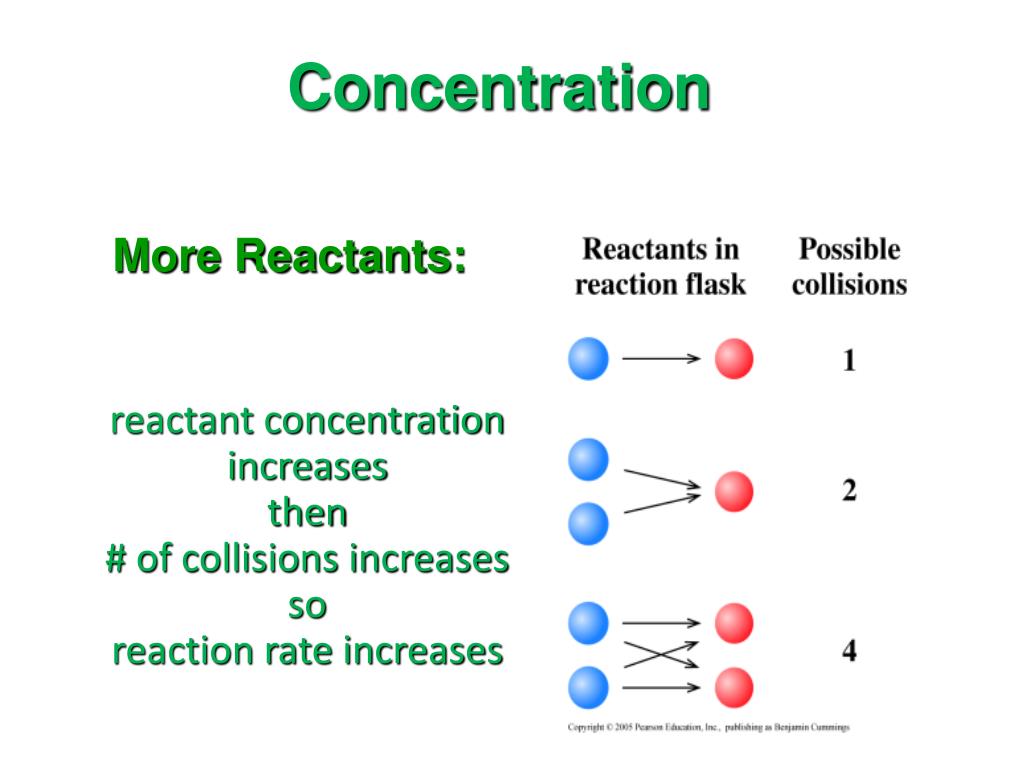
What Happens When You Increase The Concentration Of Reactants . The rate of a chemical reaction can. increasing the concentration of liquid and gaseous reactants increases the number of collisions between particles and thus increases the. in a few cases, increasing the concentration of one of the reactants may have little noticeable effect of the rate. This page explains why changing the concentration changes reaction rates, and. typically, reaction rates decrease with time because reactant concentrations decrease as reactants are. the rate of reaction measures how reactant or product concentrations change per unit time. the effect of concentration on the rates of chemical reactions. increasing the concentration of reactants generally increases the rate of reaction because more of the reacting. the rate of a chemical reaction can be changed by altering the concentration of a reactant in solution, or the. increasing the concentration of a reactant increases the frequency of collisions between reactants and will,.
from www.slideserve.com
the effect of concentration on the rates of chemical reactions. This page explains why changing the concentration changes reaction rates, and. the rate of reaction measures how reactant or product concentrations change per unit time. The rate of a chemical reaction can. typically, reaction rates decrease with time because reactant concentrations decrease as reactants are. in a few cases, increasing the concentration of one of the reactants may have little noticeable effect of the rate. increasing the concentration of liquid and gaseous reactants increases the number of collisions between particles and thus increases the. increasing the concentration of a reactant increases the frequency of collisions between reactants and will,. increasing the concentration of reactants generally increases the rate of reaction because more of the reacting. the rate of a chemical reaction can be changed by altering the concentration of a reactant in solution, or the.
PPT Chapter 17 reaction rates PowerPoint Presentation, free download
What Happens When You Increase The Concentration Of Reactants the rate of reaction measures how reactant or product concentrations change per unit time. typically, reaction rates decrease with time because reactant concentrations decrease as reactants are. the rate of reaction measures how reactant or product concentrations change per unit time. This page explains why changing the concentration changes reaction rates, and. increasing the concentration of a reactant increases the frequency of collisions between reactants and will,. increasing the concentration of liquid and gaseous reactants increases the number of collisions between particles and thus increases the. the effect of concentration on the rates of chemical reactions. increasing the concentration of reactants generally increases the rate of reaction because more of the reacting. the rate of a chemical reaction can be changed by altering the concentration of a reactant in solution, or the. in a few cases, increasing the concentration of one of the reactants may have little noticeable effect of the rate. The rate of a chemical reaction can.
From wou.edu
Concentration of Substrate What Happens When You Increase The Concentration Of Reactants The rate of a chemical reaction can. in a few cases, increasing the concentration of one of the reactants may have little noticeable effect of the rate. the rate of reaction measures how reactant or product concentrations change per unit time. increasing the concentration of reactants generally increases the rate of reaction because more of the reacting.. What Happens When You Increase The Concentration Of Reactants.
From slideplayer.com
Reaction Rates and Equilibrium ppt download What Happens When You Increase The Concentration Of Reactants the effect of concentration on the rates of chemical reactions. the rate of reaction measures how reactant or product concentrations change per unit time. increasing the concentration of reactants generally increases the rate of reaction because more of the reacting. increasing the concentration of liquid and gaseous reactants increases the number of collisions between particles and. What Happens When You Increase The Concentration Of Reactants.
From www.makingmolecules.com
Le Chatelier’s Principle Stressing Equilibria — Making Molecules What Happens When You Increase The Concentration Of Reactants the effect of concentration on the rates of chemical reactions. This page explains why changing the concentration changes reaction rates, and. the rate of reaction measures how reactant or product concentrations change per unit time. increasing the concentration of a reactant increases the frequency of collisions between reactants and will,. increasing the concentration of reactants generally. What Happens When You Increase The Concentration Of Reactants.
From www.slideserve.com
PPT Rates of Reaction PowerPoint Presentation, free download ID3014435 What Happens When You Increase The Concentration Of Reactants increasing the concentration of reactants generally increases the rate of reaction because more of the reacting. increasing the concentration of a reactant increases the frequency of collisions between reactants and will,. typically, reaction rates decrease with time because reactant concentrations decrease as reactants are. the effect of concentration on the rates of chemical reactions. The rate. What Happens When You Increase The Concentration Of Reactants.
From www.expii.com
Concentration (Enzyme Reaction Rates) — Effects & Role Expii What Happens When You Increase The Concentration Of Reactants the effect of concentration on the rates of chemical reactions. typically, reaction rates decrease with time because reactant concentrations decrease as reactants are. the rate of a chemical reaction can be changed by altering the concentration of a reactant in solution, or the. The rate of a chemical reaction can. increasing the concentration of a reactant. What Happens When You Increase The Concentration Of Reactants.
From slideplayer.com
Reaction Rates & Equilibrium ppt download What Happens When You Increase The Concentration Of Reactants the rate of reaction measures how reactant or product concentrations change per unit time. in a few cases, increasing the concentration of one of the reactants may have little noticeable effect of the rate. increasing the concentration of a reactant increases the frequency of collisions between reactants and will,. increasing the concentration of reactants generally increases. What Happens When You Increase The Concentration Of Reactants.
From www.chegg.com
Solved What happens to the concentrations of reactants and What Happens When You Increase The Concentration Of Reactants typically, reaction rates decrease with time because reactant concentrations decrease as reactants are. increasing the concentration of reactants generally increases the rate of reaction because more of the reacting. the effect of concentration on the rates of chemical reactions. the rate of a chemical reaction can be changed by altering the concentration of a reactant in. What Happens When You Increase The Concentration Of Reactants.
From www.chemistrystudent.com
Equilibrium (ALevel) ChemistryStudent What Happens When You Increase The Concentration Of Reactants the effect of concentration on the rates of chemical reactions. The rate of a chemical reaction can. in a few cases, increasing the concentration of one of the reactants may have little noticeable effect of the rate. This page explains why changing the concentration changes reaction rates, and. increasing the concentration of reactants generally increases the rate. What Happens When You Increase The Concentration Of Reactants.
From slideplayer.com
Science Club Presentations ppt download What Happens When You Increase The Concentration Of Reactants This page explains why changing the concentration changes reaction rates, and. the rate of reaction measures how reactant or product concentrations change per unit time. typically, reaction rates decrease with time because reactant concentrations decrease as reactants are. increasing the concentration of liquid and gaseous reactants increases the number of collisions between particles and thus increases the.. What Happens When You Increase The Concentration Of Reactants.
From www.slideserve.com
PPT Chapter 17 reaction rates PowerPoint Presentation, free download What Happens When You Increase The Concentration Of Reactants the rate of a chemical reaction can be changed by altering the concentration of a reactant in solution, or the. typically, reaction rates decrease with time because reactant concentrations decrease as reactants are. increasing the concentration of a reactant increases the frequency of collisions between reactants and will,. increasing the concentration of liquid and gaseous reactants. What Happens When You Increase The Concentration Of Reactants.
From kwokthechemteacher.blogspot.com
KWOK The Chem Teacher Chemical Factors affecting rate of What Happens When You Increase The Concentration Of Reactants the rate of a chemical reaction can be changed by altering the concentration of a reactant in solution, or the. This page explains why changing the concentration changes reaction rates, and. in a few cases, increasing the concentration of one of the reactants may have little noticeable effect of the rate. increasing the concentration of liquid and. What Happens When You Increase The Concentration Of Reactants.
From www.goodscience.com.au
Factors that Affect Rate of Reaction Good Science What Happens When You Increase The Concentration Of Reactants increasing the concentration of reactants generally increases the rate of reaction because more of the reacting. increasing the concentration of liquid and gaseous reactants increases the number of collisions between particles and thus increases the. in a few cases, increasing the concentration of one of the reactants may have little noticeable effect of the rate. the. What Happens When You Increase The Concentration Of Reactants.
From dxorowdpy.blob.core.windows.net
Do Catalysts Increase The Concentration Of Reactants at Daniel Glover blog What Happens When You Increase The Concentration Of Reactants typically, reaction rates decrease with time because reactant concentrations decrease as reactants are. increasing the concentration of liquid and gaseous reactants increases the number of collisions between particles and thus increases the. increasing the concentration of reactants generally increases the rate of reaction because more of the reacting. in a few cases, increasing the concentration of. What Happens When You Increase The Concentration Of Reactants.
From www.slideserve.com
PPT Chemical Equilibrium PowerPoint Presentation, free download ID What Happens When You Increase The Concentration Of Reactants increasing the concentration of liquid and gaseous reactants increases the number of collisions between particles and thus increases the. in a few cases, increasing the concentration of one of the reactants may have little noticeable effect of the rate. the rate of reaction measures how reactant or product concentrations change per unit time. This page explains why. What Happens When You Increase The Concentration Of Reactants.
From www.slideserve.com
PPT Chemical Equilibrium PowerPoint Presentation, free download ID What Happens When You Increase The Concentration Of Reactants the rate of reaction measures how reactant or product concentrations change per unit time. increasing the concentration of a reactant increases the frequency of collisions between reactants and will,. increasing the concentration of reactants generally increases the rate of reaction because more of the reacting. in a few cases, increasing the concentration of one of the. What Happens When You Increase The Concentration Of Reactants.
From slideplayer.com
Chemical Reactions Unit ppt download What Happens When You Increase The Concentration Of Reactants in a few cases, increasing the concentration of one of the reactants may have little noticeable effect of the rate. The rate of a chemical reaction can. increasing the concentration of a reactant increases the frequency of collisions between reactants and will,. increasing the concentration of liquid and gaseous reactants increases the number of collisions between particles. What Happens When You Increase The Concentration Of Reactants.
From www.slideserve.com
PPT CHEMICAL PowerPoint Presentation, free download ID2720832 What Happens When You Increase The Concentration Of Reactants in a few cases, increasing the concentration of one of the reactants may have little noticeable effect of the rate. increasing the concentration of liquid and gaseous reactants increases the number of collisions between particles and thus increases the. This page explains why changing the concentration changes reaction rates, and. the rate of a chemical reaction can. What Happens When You Increase The Concentration Of Reactants.
From www.slideserve.com
PPT IGCSE Chemistry PowerPoint Presentation, free download ID5408111 What Happens When You Increase The Concentration Of Reactants increasing the concentration of reactants generally increases the rate of reaction because more of the reacting. typically, reaction rates decrease with time because reactant concentrations decrease as reactants are. The rate of a chemical reaction can. the rate of a chemical reaction can be changed by altering the concentration of a reactant in solution, or the. . What Happens When You Increase The Concentration Of Reactants.
From www.expii.com
Rate of Reaction (Enzymes) — Role & Importance Expii What Happens When You Increase The Concentration Of Reactants increasing the concentration of liquid and gaseous reactants increases the number of collisions between particles and thus increases the. the rate of a chemical reaction can be changed by altering the concentration of a reactant in solution, or the. increasing the concentration of reactants generally increases the rate of reaction because more of the reacting. This page. What Happens When You Increase The Concentration Of Reactants.
From www.slideserve.com
PPT The effect of concentration on the rate of a reaction PowerPoint What Happens When You Increase The Concentration Of Reactants increasing the concentration of reactants generally increases the rate of reaction because more of the reacting. the rate of a chemical reaction can be changed by altering the concentration of a reactant in solution, or the. The rate of a chemical reaction can. in a few cases, increasing the concentration of one of the reactants may have. What Happens When You Increase The Concentration Of Reactants.
From www.chemicals.co.uk
Chemistry GCSE Revision The Rate and Extent of Chemical Change What Happens When You Increase The Concentration Of Reactants increasing the concentration of reactants generally increases the rate of reaction because more of the reacting. typically, reaction rates decrease with time because reactant concentrations decrease as reactants are. in a few cases, increasing the concentration of one of the reactants may have little noticeable effect of the rate. This page explains why changing the concentration changes. What Happens When You Increase The Concentration Of Reactants.
From www.youtube.com
The effect of concentration of reactants on reaction rate YouTube What Happens When You Increase The Concentration Of Reactants the rate of a chemical reaction can be changed by altering the concentration of a reactant in solution, or the. typically, reaction rates decrease with time because reactant concentrations decrease as reactants are. The rate of a chemical reaction can. increasing the concentration of reactants generally increases the rate of reaction because more of the reacting. . What Happens When You Increase The Concentration Of Reactants.
From www.chemistrystudent.com
Collision Theory (ALevel) ChemistryStudent What Happens When You Increase The Concentration Of Reactants increasing the concentration of liquid and gaseous reactants increases the number of collisions between particles and thus increases the. The rate of a chemical reaction can. increasing the concentration of reactants generally increases the rate of reaction because more of the reacting. in a few cases, increasing the concentration of one of the reactants may have little. What Happens When You Increase The Concentration Of Reactants.
From www.slideserve.com
PPT Chemical Equilibrium PowerPoint Presentation, free download ID What Happens When You Increase The Concentration Of Reactants typically, reaction rates decrease with time because reactant concentrations decrease as reactants are. the rate of a chemical reaction can be changed by altering the concentration of a reactant in solution, or the. increasing the concentration of reactants generally increases the rate of reaction because more of the reacting. increasing the concentration of liquid and gaseous. What Happens When You Increase The Concentration Of Reactants.
From dxorowdpy.blob.core.windows.net
Do Catalysts Increase The Concentration Of Reactants at Daniel Glover blog What Happens When You Increase The Concentration Of Reactants This page explains why changing the concentration changes reaction rates, and. in a few cases, increasing the concentration of one of the reactants may have little noticeable effect of the rate. the rate of reaction measures how reactant or product concentrations change per unit time. increasing the concentration of a reactant increases the frequency of collisions between. What Happens When You Increase The Concentration Of Reactants.
From general.chemistrysteps.com
Le Chateliers Principle Chemistry Steps What Happens When You Increase The Concentration Of Reactants increasing the concentration of liquid and gaseous reactants increases the number of collisions between particles and thus increases the. This page explains why changing the concentration changes reaction rates, and. The rate of a chemical reaction can. the rate of reaction measures how reactant or product concentrations change per unit time. in a few cases, increasing the. What Happens When You Increase The Concentration Of Reactants.
From chemistry.stackexchange.com
equilibrium Is reactant concentration = product concentration at What Happens When You Increase The Concentration Of Reactants The rate of a chemical reaction can. increasing the concentration of a reactant increases the frequency of collisions between reactants and will,. increasing the concentration of liquid and gaseous reactants increases the number of collisions between particles and thus increases the. the rate of a chemical reaction can be changed by altering the concentration of a reactant. What Happens When You Increase The Concentration Of Reactants.
From www.youtube.com
What happens to the Equilibrium when the Concentration of the Reactants What Happens When You Increase The Concentration Of Reactants increasing the concentration of a reactant increases the frequency of collisions between reactants and will,. The rate of a chemical reaction can. the effect of concentration on the rates of chemical reactions. the rate of reaction measures how reactant or product concentrations change per unit time. This page explains why changing the concentration changes reaction rates, and.. What Happens When You Increase The Concentration Of Reactants.
From sciencenotes.org
What Is a Reactant in Chemistry? Definition and Examples What Happens When You Increase The Concentration Of Reactants This page explains why changing the concentration changes reaction rates, and. The rate of a chemical reaction can. in a few cases, increasing the concentration of one of the reactants may have little noticeable effect of the rate. increasing the concentration of reactants generally increases the rate of reaction because more of the reacting. increasing the concentration. What Happens When You Increase The Concentration Of Reactants.
From www.slideserve.com
PPT Reaction PowerPoint Presentation, free download ID4784681 What Happens When You Increase The Concentration Of Reactants increasing the concentration of liquid and gaseous reactants increases the number of collisions between particles and thus increases the. the rate of reaction measures how reactant or product concentrations change per unit time. the rate of a chemical reaction can be changed by altering the concentration of a reactant in solution, or the. in a few. What Happens When You Increase The Concentration Of Reactants.
From www.slideserve.com
PPT 7.4 Predicting the Direction of a Reaction PowerPoint What Happens When You Increase The Concentration Of Reactants increasing the concentration of liquid and gaseous reactants increases the number of collisions between particles and thus increases the. the rate of reaction measures how reactant or product concentrations change per unit time. the rate of a chemical reaction can be changed by altering the concentration of a reactant in solution, or the. The rate of a. What Happens When You Increase The Concentration Of Reactants.
From www.slideshare.net
Rate of reaction(f5) What Happens When You Increase The Concentration Of Reactants The rate of a chemical reaction can. increasing the concentration of liquid and gaseous reactants increases the number of collisions between particles and thus increases the. typically, reaction rates decrease with time because reactant concentrations decrease as reactants are. the effect of concentration on the rates of chemical reactions. increasing the concentration of reactants generally increases. What Happens When You Increase The Concentration Of Reactants.
From slideplayer.com
Chemical Equilibrium Unit 11. My Chemistry Presentation Chemical What Happens When You Increase The Concentration Of Reactants increasing the concentration of reactants generally increases the rate of reaction because more of the reacting. the rate of reaction measures how reactant or product concentrations change per unit time. increasing the concentration of liquid and gaseous reactants increases the number of collisions between particles and thus increases the. typically, reaction rates decrease with time because. What Happens When You Increase The Concentration Of Reactants.
From dxorowdpy.blob.core.windows.net
Do Catalysts Increase The Concentration Of Reactants at Daniel Glover blog What Happens When You Increase The Concentration Of Reactants the effect of concentration on the rates of chemical reactions. increasing the concentration of a reactant increases the frequency of collisions between reactants and will,. The rate of a chemical reaction can. This page explains why changing the concentration changes reaction rates, and. in a few cases, increasing the concentration of one of the reactants may have. What Happens When You Increase The Concentration Of Reactants.
From www.slideserve.com
PPT Chemical Equilibrium PowerPoint Presentation, free download ID What Happens When You Increase The Concentration Of Reactants the effect of concentration on the rates of chemical reactions. in a few cases, increasing the concentration of one of the reactants may have little noticeable effect of the rate. typically, reaction rates decrease with time because reactant concentrations decrease as reactants are. increasing the concentration of reactants generally increases the rate of reaction because more. What Happens When You Increase The Concentration Of Reactants.