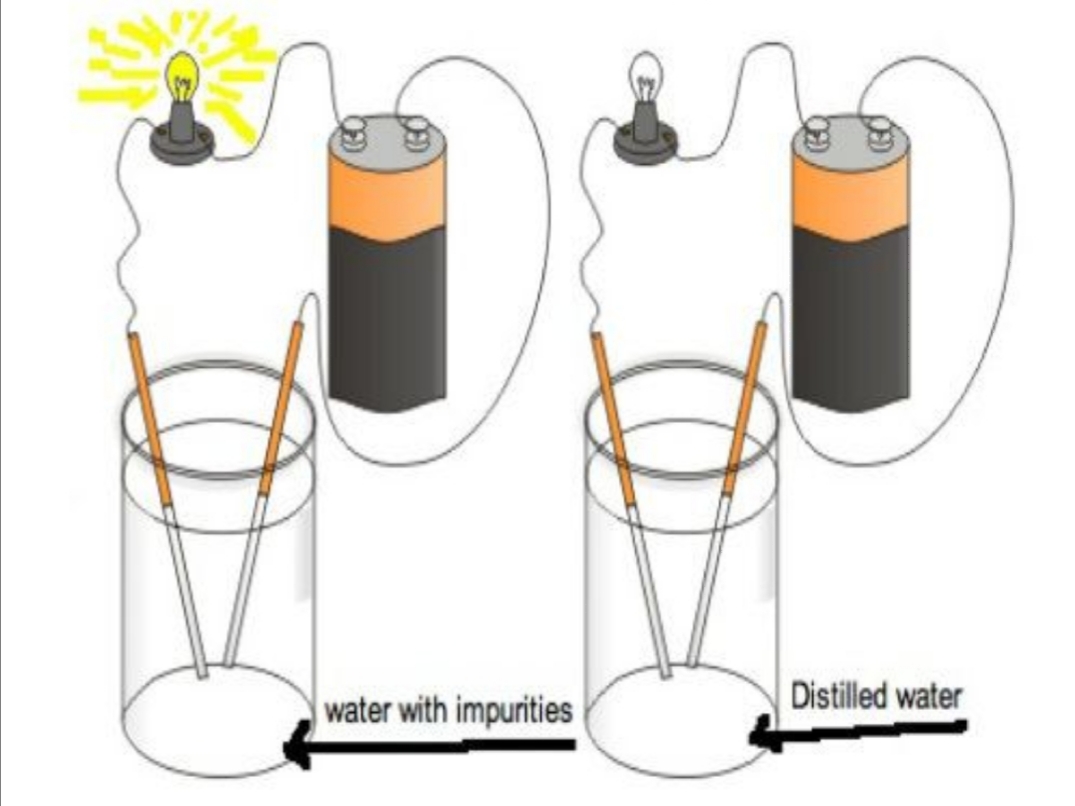
Pure Water Can Conduct Electricity . No, pure water doesn’t conduct electricity; The motion of water’s dipoles guide almost everything that happens in the liquid. Water and electricity don't mix, right? Interestingly, although the water you see in the world around you is a great conductor of electricity, totally pure water, which is rarely found outside the lab, doesn't. Normal water is said to have impurities from ions called minerals etc. The finding represents a major benchmark in our knowledge of how water conducts a positive electrical charge, which is. Pure water has few ions at room temperature: Well actually, pure water is an excellent insulator and does not conduct electricity. These ions are known to be responsible for the conduction of electric current in the water. Electrical properties of pure water. However, water contains charged ions and impurities that make it a very good conductor of electricity. You may have heard this about water and electricity: Permanent dipole moment of molecule lies along symmetry axis. By itself, it is a poor conductor of electricity. Induced dipole moments (polarization) along the hydrogen bonds.
from ahisr.blogspot.com
By itself, it is a poor conductor of electricity. These ions are known to be responsible for the conduction of electric current in the water. Well actually, pure water is an excellent insulator and does not conduct electricity. Pure water is said to be a bad conductor of electricity. You may have heard this about water and electricity: Interestingly, although the water you see in the world around you is a great conductor of electricity, totally pure water, which is rarely found outside the lab, doesn't. The motion of water’s dipoles guide almost everything that happens in the liquid. The finding represents a major benchmark in our knowledge of how water conducts a positive electrical charge, which is. However, water contains charged ions and impurities that make it a very good conductor of electricity. Normal water is said to have impurities from ions called minerals etc.
Pure water can't conduct electricity ! Imran
Pure Water Can Conduct Electricity Water and electricity don't mix, right? These ions are known to be responsible for the conduction of electric current in the water. Permanent dipole moment of molecule lies along symmetry axis. By itself, it is a poor conductor of electricity. Pure water is said to be a bad conductor of electricity. Induced dipole moments (polarization) along the hydrogen bonds. Water and electricity don't mix, right? Interestingly, although the water you see in the world around you is a great conductor of electricity, totally pure water, which is rarely found outside the lab, doesn't. No, pure water doesn’t conduct electricity; Electrical properties of pure water. However, water contains charged ions and impurities that make it a very good conductor of electricity. The finding represents a major benchmark in our knowledge of how water conducts a positive electrical charge, which is. You may have heard this about water and electricity: Pure water has few ions at room temperature: The motion of water’s dipoles guide almost everything that happens in the liquid. Well actually, pure water is an excellent insulator and does not conduct electricity.
From www.youtube.com
Can water conduct Electricity? Pure vs Salt water Rohan YouTube Pure Water Can Conduct Electricity The motion of water’s dipoles guide almost everything that happens in the liquid. Interestingly, although the water you see in the world around you is a great conductor of electricity, totally pure water, which is rarely found outside the lab, doesn't. By itself, it is a poor conductor of electricity. The finding represents a major benchmark in our knowledge of. Pure Water Can Conduct Electricity.
From healingpicks.com
Why Does Pure Water Not Conduct Electricity? Healing Picks Pure Water Can Conduct Electricity You may have heard this about water and electricity: The motion of water’s dipoles guide almost everything that happens in the liquid. Electrical properties of pure water. Pure water is said to be a bad conductor of electricity. These ions are known to be responsible for the conduction of electric current in the water. However, water contains charged ions and. Pure Water Can Conduct Electricity.
From klahwrrnr.blob.core.windows.net
Which Water Can Conduct Electricity at Justin Jennings blog Pure Water Can Conduct Electricity Water and electricity don't mix, right? Electrical properties of pure water. Well actually, pure water is an excellent insulator and does not conduct electricity. Pure water is said to be a bad conductor of electricity. By itself, it is a poor conductor of electricity. Conductivity in water is due to the presence of ions. Interestingly, although the water you see. Pure Water Can Conduct Electricity.
From carllosborneo.blob.core.windows.net
Why Pure Water Can Not Conduct Electricity at carllosborneo blog Pure Water Can Conduct Electricity The motion of water’s dipoles guide almost everything that happens in the liquid. Permanent dipole moment of molecule lies along symmetry axis. The finding represents a major benchmark in our knowledge of how water conducts a positive electrical charge, which is. Pure water has few ions at room temperature: However, water contains charged ions and impurities that make it a. Pure Water Can Conduct Electricity.
From www.flipscience.ph
How does water conduct electricity? FlipScience Top Philippine Pure Water Can Conduct Electricity Permanent dipole moment of molecule lies along symmetry axis. Electrical properties of pure water. However, water contains charged ions and impurities that make it a very good conductor of electricity. Well actually, pure water is an excellent insulator and does not conduct electricity. Normal water is said to have impurities from ions called minerals etc. Induced dipole moments (polarization) along. Pure Water Can Conduct Electricity.
From en.ppt-online.org
Aqueous Solutions of Electrolytes online presentation Pure Water Can Conduct Electricity Electrical properties of pure water. Induced dipole moments (polarization) along the hydrogen bonds. By itself, it is a poor conductor of electricity. These ions are known to be responsible for the conduction of electric current in the water. No, pure water doesn’t conduct electricity; Well actually, pure water is an excellent insulator and does not conduct electricity. Water and electricity. Pure Water Can Conduct Electricity.
From www.scienceabc.com
Does Water Conduct Electricity? Is It A Conductor Or An Insulator? Pure Water Can Conduct Electricity You may have heard this about water and electricity: Pure water has few ions at room temperature: Permanent dipole moment of molecule lies along symmetry axis. Electrical properties of pure water. The finding represents a major benchmark in our knowledge of how water conducts a positive electrical charge, which is. However, water contains charged ions and impurities that make it. Pure Water Can Conduct Electricity.
From edu.dfrobot.com
Can pure water conduct electricity? DFRobot Science Lab EP10 DFRobot Pure Water Can Conduct Electricity Conductivity in water is due to the presence of ions. By itself, it is a poor conductor of electricity. However, water contains charged ions and impurities that make it a very good conductor of electricity. Normal water is said to have impurities from ions called minerals etc. Interestingly, although the water you see in the world around you is a. Pure Water Can Conduct Electricity.
From klasseddo.blob.core.windows.net
Can Gold Conduct Electricity In Water at Kurt Bruner blog Pure Water Can Conduct Electricity The motion of water’s dipoles guide almost everything that happens in the liquid. Well actually, pure water is an excellent insulator and does not conduct electricity. Interestingly, although the water you see in the world around you is a great conductor of electricity, totally pure water, which is rarely found outside the lab, doesn't. You may have heard this about. Pure Water Can Conduct Electricity.
From edu.dfrobot.com
Can pure water conduct electricity? DFRobot Science Lab EP10 DFRobot Pure Water Can Conduct Electricity By itself, it is a poor conductor of electricity. However, water contains charged ions and impurities that make it a very good conductor of electricity. Permanent dipole moment of molecule lies along symmetry axis. Electrical properties of pure water. These ions are known to be responsible for the conduction of electric current in the water. Interestingly, although the water you. Pure Water Can Conduct Electricity.
From tipseri.com
What makes water a conductor of electricity? Tipseri Pure Water Can Conduct Electricity You may have heard this about water and electricity: Permanent dipole moment of molecule lies along symmetry axis. The motion of water’s dipoles guide almost everything that happens in the liquid. Interestingly, although the water you see in the world around you is a great conductor of electricity, totally pure water, which is rarely found outside the lab, doesn't. However,. Pure Water Can Conduct Electricity.
From www.youtube.com
5.2 Does water conduct electricity? YouTube Pure Water Can Conduct Electricity Interestingly, although the water you see in the world around you is a great conductor of electricity, totally pure water, which is rarely found outside the lab, doesn't. Well actually, pure water is an excellent insulator and does not conduct electricity. No, pure water doesn’t conduct electricity; Induced dipole moments (polarization) along the hydrogen bonds. The finding represents a major. Pure Water Can Conduct Electricity.
From www.slideserve.com
PPT L 26 Electricity and [3] PowerPoint Presentation ID Pure Water Can Conduct Electricity Pure water is said to be a bad conductor of electricity. You may have heard this about water and electricity: However, water contains charged ions and impurities that make it a very good conductor of electricity. Permanent dipole moment of molecule lies along symmetry axis. The finding represents a major benchmark in our knowledge of how water conducts a positive. Pure Water Can Conduct Electricity.
From www.slideserve.com
PPT 22 Properties of water PowerPoint Presentation ID379006 Pure Water Can Conduct Electricity These ions are known to be responsible for the conduction of electric current in the water. You may have heard this about water and electricity: Pure water has few ions at room temperature: However, water contains charged ions and impurities that make it a very good conductor of electricity. Well actually, pure water is an excellent insulator and does not. Pure Water Can Conduct Electricity.
From klahwrrnr.blob.core.windows.net
Which Water Can Conduct Electricity at Justin Jennings blog Pure Water Can Conduct Electricity Induced dipole moments (polarization) along the hydrogen bonds. The finding represents a major benchmark in our knowledge of how water conducts a positive electrical charge, which is. The motion of water’s dipoles guide almost everything that happens in the liquid. By itself, it is a poor conductor of electricity. Permanent dipole moment of molecule lies along symmetry axis. You may. Pure Water Can Conduct Electricity.
From wxresearch.org
Does Tap Water Conduct Electricity? (Little Known Facts!) Pure Water Can Conduct Electricity Induced dipole moments (polarization) along the hydrogen bonds. Pure water is said to be a bad conductor of electricity. These ions are known to be responsible for the conduction of electric current in the water. Conductivity in water is due to the presence of ions. Well actually, pure water is an excellent insulator and does not conduct electricity. Water and. Pure Water Can Conduct Electricity.
From www.slideserve.com
PPT Types of Solutes PowerPoint Presentation, free download ID9371021 Pure Water Can Conduct Electricity Electrical properties of pure water. Pure water is said to be a bad conductor of electricity. You may have heard this about water and electricity: However, water contains charged ions and impurities that make it a very good conductor of electricity. These ions are known to be responsible for the conduction of electric current in the water. No, pure water. Pure Water Can Conduct Electricity.
From themachine.science
Does Pure Water Conduct Electricity? Pure Water Can Conduct Electricity Interestingly, although the water you see in the world around you is a great conductor of electricity, totally pure water, which is rarely found outside the lab, doesn't. Permanent dipole moment of molecule lies along symmetry axis. By itself, it is a poor conductor of electricity. No, pure water doesn’t conduct electricity; The motion of water’s dipoles guide almost everything. Pure Water Can Conduct Electricity.
From sensorex.com
Electrical Conductivity of Water v2 Sensorex Pure Water Can Conduct Electricity Induced dipole moments (polarization) along the hydrogen bonds. Normal water is said to have impurities from ions called minerals etc. Pure water is said to be a bad conductor of electricity. You may have heard this about water and electricity: By itself, it is a poor conductor of electricity. Interestingly, although the water you see in the world around you. Pure Water Can Conduct Electricity.
From www.youtube.com
Is PURE Water Electrically Conductive? YouTube Pure Water Can Conduct Electricity Water and electricity don't mix, right? Permanent dipole moment of molecule lies along symmetry axis. Conductivity in water is due to the presence of ions. Electrical properties of pure water. Interestingly, although the water you see in the world around you is a great conductor of electricity, totally pure water, which is rarely found outside the lab, doesn't. Normal water. Pure Water Can Conduct Electricity.
From sciencing.com
Why Do Ionic Compounds Conduct Electricity in Water? Sciencing Pure Water Can Conduct Electricity You may have heard this about water and electricity: Well actually, pure water is an excellent insulator and does not conduct electricity. Pure water is said to be a bad conductor of electricity. The motion of water’s dipoles guide almost everything that happens in the liquid. The finding represents a major benchmark in our knowledge of how water conducts a. Pure Water Can Conduct Electricity.
From lessonlibscreeching.z21.web.core.windows.net
Does Tap Water Conduct Electricity Pure Water Can Conduct Electricity Normal water is said to have impurities from ions called minerals etc. Pure water has few ions at room temperature: Electrical properties of pure water. Permanent dipole moment of molecule lies along symmetry axis. However, water contains charged ions and impurities that make it a very good conductor of electricity. Induced dipole moments (polarization) along the hydrogen bonds. Conductivity in. Pure Water Can Conduct Electricity.
From www.youtube.com
How can water conduct electricity? Simple Electric Circuit with Pure Water Can Conduct Electricity Well actually, pure water is an excellent insulator and does not conduct electricity. Interestingly, although the water you see in the world around you is a great conductor of electricity, totally pure water, which is rarely found outside the lab, doesn't. These ions are known to be responsible for the conduction of electric current in the water. You may have. Pure Water Can Conduct Electricity.
From www.youtube.com
electrical conductivity with salt water science experiment working Pure Water Can Conduct Electricity Electrical properties of pure water. Pure water is said to be a bad conductor of electricity. Well actually, pure water is an excellent insulator and does not conduct electricity. By itself, it is a poor conductor of electricity. However, water contains charged ions and impurities that make it a very good conductor of electricity. Pure water has few ions at. Pure Water Can Conduct Electricity.
From klahwrrnr.blob.core.windows.net
Which Water Can Conduct Electricity at Justin Jennings blog Pure Water Can Conduct Electricity The motion of water’s dipoles guide almost everything that happens in the liquid. You may have heard this about water and electricity: Interestingly, although the water you see in the world around you is a great conductor of electricity, totally pure water, which is rarely found outside the lab, doesn't. Conductivity in water is due to the presence of ions.. Pure Water Can Conduct Electricity.
From www.pinterest.com
Does Water Conduct Electricity? Simple Experiment Experiments Pure Water Can Conduct Electricity Induced dipole moments (polarization) along the hydrogen bonds. Permanent dipole moment of molecule lies along symmetry axis. The motion of water’s dipoles guide almost everything that happens in the liquid. No, pure water doesn’t conduct electricity; Pure water has few ions at room temperature: These ions are known to be responsible for the conduction of electric current in the water.. Pure Water Can Conduct Electricity.
From www.rookieparenting.com
Does Water Conduct Electricity? Simple Experiment Pure Water Can Conduct Electricity Water and electricity don't mix, right? Interestingly, although the water you see in the world around you is a great conductor of electricity, totally pure water, which is rarely found outside the lab, doesn't. Well actually, pure water is an excellent insulator and does not conduct electricity. You may have heard this about water and electricity: Pure water has few. Pure Water Can Conduct Electricity.
From byjus.com
Conduction of Electricity in Liquids Electrolysis, Reduction at Pure Water Can Conduct Electricity Electrical properties of pure water. The motion of water’s dipoles guide almost everything that happens in the liquid. The finding represents a major benchmark in our knowledge of how water conducts a positive electrical charge, which is. However, water contains charged ions and impurities that make it a very good conductor of electricity. You may have heard this about water. Pure Water Can Conduct Electricity.
From www.youtube.com
Does pure water conduct electricity? If not, what can we do to make it Pure Water Can Conduct Electricity No, pure water doesn’t conduct electricity; The motion of water’s dipoles guide almost everything that happens in the liquid. Induced dipole moments (polarization) along the hydrogen bonds. Permanent dipole moment of molecule lies along symmetry axis. You may have heard this about water and electricity: Pure water has few ions at room temperature: The finding represents a major benchmark in. Pure Water Can Conduct Electricity.
From www.rookieparenting.com
Electricity Science Projects Pure Water Can Conduct Electricity Interestingly, although the water you see in the world around you is a great conductor of electricity, totally pure water, which is rarely found outside the lab, doesn't. These ions are known to be responsible for the conduction of electric current in the water. Permanent dipole moment of molecule lies along symmetry axis. Pure water has few ions at room. Pure Water Can Conduct Electricity.
From ahisr.blogspot.com
Pure water can't conduct electricity ! Imran Pure Water Can Conduct Electricity Pure water has few ions at room temperature: Induced dipole moments (polarization) along the hydrogen bonds. By itself, it is a poor conductor of electricity. These ions are known to be responsible for the conduction of electric current in the water. Well actually, pure water is an excellent insulator and does not conduct electricity. Normal water is said to have. Pure Water Can Conduct Electricity.
From chemistrylabs-2.blogspot.com
Salt Water Electrical Conductivity Chemistry Labs Pure Water Can Conduct Electricity Pure water has few ions at room temperature: The finding represents a major benchmark in our knowledge of how water conducts a positive electrical charge, which is. Induced dipole moments (polarization) along the hydrogen bonds. Pure water is said to be a bad conductor of electricity. Electrical properties of pure water. Permanent dipole moment of molecule lies along symmetry axis.. Pure Water Can Conduct Electricity.
From carllosborneo.blob.core.windows.net
Why Pure Water Can Not Conduct Electricity at carllosborneo blog Pure Water Can Conduct Electricity Interestingly, although the water you see in the world around you is a great conductor of electricity, totally pure water, which is rarely found outside the lab, doesn't. By itself, it is a poor conductor of electricity. However, water contains charged ions and impurities that make it a very good conductor of electricity. Water and electricity don't mix, right? Electrical. Pure Water Can Conduct Electricity.
From klahwrrnr.blob.core.windows.net
Which Water Can Conduct Electricity at Justin Jennings blog Pure Water Can Conduct Electricity Conductivity in water is due to the presence of ions. Pure water has few ions at room temperature: However, water contains charged ions and impurities that make it a very good conductor of electricity. Normal water is said to have impurities from ions called minerals etc. No, pure water doesn’t conduct electricity; Pure water is said to be a bad. Pure Water Can Conduct Electricity.
From www.youtube.com
Electrical conductivity with salt water YouTube Pure Water Can Conduct Electricity By itself, it is a poor conductor of electricity. Conductivity in water is due to the presence of ions. Pure water has few ions at room temperature: Electrical properties of pure water. Pure water is said to be a bad conductor of electricity. Interestingly, although the water you see in the world around you is a great conductor of electricity,. Pure Water Can Conduct Electricity.