The Boiling Point Of Liquid Can Be Decreased By . The pressure of gas above a liquid affects the boiling point. As the altitude increases, the boiling. In an open system this is called atmospheric pressure. Some liquids have such high normal boiling points that they begin to decompose before distillation can be carried out. As the altitude increases, the boiling point decreases. The dependence of the boiling point on the external pressure can often be very useful. Equal to one atmosphere, the boiling point of a liquid is called the normal boiling point. The boiling point is the temperature at which the vapor pressure of a liquid is equal to the external pressure. The boiling point is the temperature at which the vapor pressure of a liquid is equal to the external pressure. Chemists often purify liquids by boiling them and collecting the vapor, a process known as distillation. Boiling point of a liquid can be increased by increasing the external pressure on the liquid, whereas it can be decreased by decreasing the. Less than one atmosphere, the boiling point of the liquid is lower than its normal boiling point. Lowering the surrounding pressure, either by increasing altitude or. Using a closed system to artificially increase that pressure will raise the boiling point of a liquid.
from www.vedantu.com
Using a closed system to artificially increase that pressure will raise the boiling point of a liquid. Lowering the surrounding pressure, either by increasing altitude or. The dependence of the boiling point on the external pressure can often be very useful. Less than one atmosphere, the boiling point of the liquid is lower than its normal boiling point. The pressure of gas above a liquid affects the boiling point. The boiling point is the temperature at which the vapor pressure of a liquid is equal to the external pressure. Chemists often purify liquids by boiling them and collecting the vapor, a process known as distillation. The boiling point is the temperature at which the vapor pressure of a liquid is equal to the external pressure. Some liquids have such high normal boiling points that they begin to decompose before distillation can be carried out. As the altitude increases, the boiling point decreases.
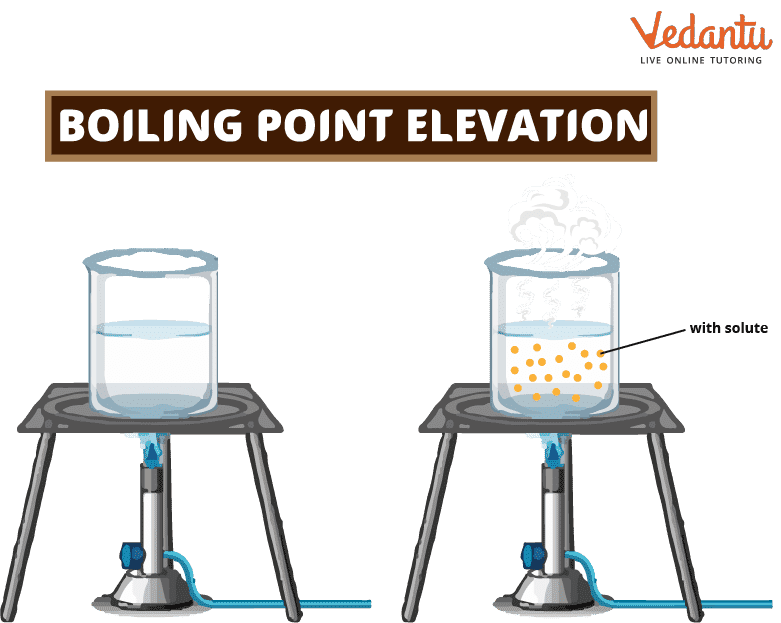
Boiling Point Elevation Learn Important Terms and Concepts
The Boiling Point Of Liquid Can Be Decreased By As the altitude increases, the boiling. Less than one atmosphere, the boiling point of the liquid is lower than its normal boiling point. As the altitude increases, the boiling point decreases. The boiling point is the temperature at which the vapor pressure of a liquid is equal to the external pressure. Using a closed system to artificially increase that pressure will raise the boiling point of a liquid. Chemists often purify liquids by boiling them and collecting the vapor, a process known as distillation. Boiling point of a liquid can be increased by increasing the external pressure on the liquid, whereas it can be decreased by decreasing the. Some liquids have such high normal boiling points that they begin to decompose before distillation can be carried out. The pressure of gas above a liquid affects the boiling point. In an open system this is called atmospheric pressure. Lowering the surrounding pressure, either by increasing altitude or. The dependence of the boiling point on the external pressure can often be very useful. Equal to one atmosphere, the boiling point of a liquid is called the normal boiling point. The boiling point is the temperature at which the vapor pressure of a liquid is equal to the external pressure. As the altitude increases, the boiling.
From joixgvqec.blob.core.windows.net
Why Is The Boiling Point Of Water High at Tina Edmonds blog The Boiling Point Of Liquid Can Be Decreased By Less than one atmosphere, the boiling point of the liquid is lower than its normal boiling point. In an open system this is called atmospheric pressure. The pressure of gas above a liquid affects the boiling point. The boiling point is the temperature at which the vapor pressure of a liquid is equal to the external pressure. As the altitude. The Boiling Point Of Liquid Can Be Decreased By.
From www.slideserve.com
PPT Chapter 10 States of Matter PowerPoint Presentation, free The Boiling Point Of Liquid Can Be Decreased By Less than one atmosphere, the boiling point of the liquid is lower than its normal boiling point. Equal to one atmosphere, the boiling point of a liquid is called the normal boiling point. Using a closed system to artificially increase that pressure will raise the boiling point of a liquid. The pressure of gas above a liquid affects the boiling. The Boiling Point Of Liquid Can Be Decreased By.
From www.youtube.com
Binary Boiling Point Diagram of a LiquidLiquid Mixture YouTube The Boiling Point Of Liquid Can Be Decreased By Lowering the surrounding pressure, either by increasing altitude or. Using a closed system to artificially increase that pressure will raise the boiling point of a liquid. As the altitude increases, the boiling. Less than one atmosphere, the boiling point of the liquid is lower than its normal boiling point. The pressure of gas above a liquid affects the boiling point.. The Boiling Point Of Liquid Can Be Decreased By.
From www.coursehero.com
[Solved] 1. 2. . The normal boiling point of liquid ethane thiol is 309 The Boiling Point Of Liquid Can Be Decreased By The boiling point is the temperature at which the vapor pressure of a liquid is equal to the external pressure. Lowering the surrounding pressure, either by increasing altitude or. Chemists often purify liquids by boiling them and collecting the vapor, a process known as distillation. Boiling point of a liquid can be increased by increasing the external pressure on the. The Boiling Point Of Liquid Can Be Decreased By.
From www.numerade.com
SOLVED (6). Define distillation? boiling liquids condensing liquids The Boiling Point Of Liquid Can Be Decreased By In an open system this is called atmospheric pressure. Using a closed system to artificially increase that pressure will raise the boiling point of a liquid. As the altitude increases, the boiling. The dependence of the boiling point on the external pressure can often be very useful. Chemists often purify liquids by boiling them and collecting the vapor, a process. The Boiling Point Of Liquid Can Be Decreased By.
From www.slideserve.com
PPT Review LIQUIDS & PHASE CHANGES PowerPoint Presentation, free The Boiling Point Of Liquid Can Be Decreased By The boiling point is the temperature at which the vapor pressure of a liquid is equal to the external pressure. The boiling point is the temperature at which the vapor pressure of a liquid is equal to the external pressure. As the altitude increases, the boiling point decreases. The dependence of the boiling point on the external pressure can often. The Boiling Point Of Liquid Can Be Decreased By.
From www.youtube.com
Boiling Point of Organic Compounds YouTube The Boiling Point Of Liquid Can Be Decreased By The boiling point is the temperature at which the vapor pressure of a liquid is equal to the external pressure. Less than one atmosphere, the boiling point of the liquid is lower than its normal boiling point. Lowering the surrounding pressure, either by increasing altitude or. Using a closed system to artificially increase that pressure will raise the boiling point. The Boiling Point Of Liquid Can Be Decreased By.
From www.vedantu.com
Boiling Point Elevation Learn Important Terms and Concepts The Boiling Point Of Liquid Can Be Decreased By The boiling point is the temperature at which the vapor pressure of a liquid is equal to the external pressure. Using a closed system to artificially increase that pressure will raise the boiling point of a liquid. Less than one atmosphere, the boiling point of the liquid is lower than its normal boiling point. The boiling point is the temperature. The Boiling Point Of Liquid Can Be Decreased By.
From www.slideserve.com
PPT Chapter 13 continued… PowerPoint Presentation, free download ID The Boiling Point Of Liquid Can Be Decreased By Boiling point of a liquid can be increased by increasing the external pressure on the liquid, whereas it can be decreased by decreasing the. Using a closed system to artificially increase that pressure will raise the boiling point of a liquid. Less than one atmosphere, the boiling point of the liquid is lower than its normal boiling point. The boiling. The Boiling Point Of Liquid Can Be Decreased By.
From www.semanticscholar.org
[PDF] The boiling suppression of liquid nitrogen Semantic Scholar The Boiling Point Of Liquid Can Be Decreased By Equal to one atmosphere, the boiling point of a liquid is called the normal boiling point. The boiling point is the temperature at which the vapor pressure of a liquid is equal to the external pressure. The boiling point is the temperature at which the vapor pressure of a liquid is equal to the external pressure. The dependence of the. The Boiling Point Of Liquid Can Be Decreased By.
From www.youtube.com
Boiling Point of liquid & Factors on which boiling point depend The Boiling Point Of Liquid Can Be Decreased By The boiling point is the temperature at which the vapor pressure of a liquid is equal to the external pressure. Equal to one atmosphere, the boiling point of a liquid is called the normal boiling point. Lowering the surrounding pressure, either by increasing altitude or. In an open system this is called atmospheric pressure. Using a closed system to artificially. The Boiling Point Of Liquid Can Be Decreased By.
From engineeringstuff.co.in
What is Boiling point Engineeringstuff The Boiling Point Of Liquid Can Be Decreased By Using a closed system to artificially increase that pressure will raise the boiling point of a liquid. The boiling point is the temperature at which the vapor pressure of a liquid is equal to the external pressure. The pressure of gas above a liquid affects the boiling point. In an open system this is called atmospheric pressure. As the altitude. The Boiling Point Of Liquid Can Be Decreased By.
From general.chemistrysteps.com
Boiling Point Elevation Chemistry Steps The Boiling Point Of Liquid Can Be Decreased By As the altitude increases, the boiling. Chemists often purify liquids by boiling them and collecting the vapor, a process known as distillation. The pressure of gas above a liquid affects the boiling point. In an open system this is called atmospheric pressure. Lowering the surrounding pressure, either by increasing altitude or. Using a closed system to artificially increase that pressure. The Boiling Point Of Liquid Can Be Decreased By.
From www.worksheetsplanet.com
What is Boiling Point The Boiling Point Of Liquid Can Be Decreased By Lowering the surrounding pressure, either by increasing altitude or. Some liquids have such high normal boiling points that they begin to decompose before distillation can be carried out. Less than one atmosphere, the boiling point of the liquid is lower than its normal boiling point. Equal to one atmosphere, the boiling point of a liquid is called the normal boiling. The Boiling Point Of Liquid Can Be Decreased By.
From exotahhqc.blob.core.windows.net
Water Boils Faster At High Altitudes at Rosa Smith blog The Boiling Point Of Liquid Can Be Decreased By Some liquids have such high normal boiling points that they begin to decompose before distillation can be carried out. In an open system this is called atmospheric pressure. Less than one atmosphere, the boiling point of the liquid is lower than its normal boiling point. Chemists often purify liquids by boiling them and collecting the vapor, a process known as. The Boiling Point Of Liquid Can Be Decreased By.
From www.toppr.com
Two liquids (A) and (B) can be separated by the method of fractional The Boiling Point Of Liquid Can Be Decreased By The pressure of gas above a liquid affects the boiling point. Using a closed system to artificially increase that pressure will raise the boiling point of a liquid. Some liquids have such high normal boiling points that they begin to decompose before distillation can be carried out. In an open system this is called atmospheric pressure. Chemists often purify liquids. The Boiling Point Of Liquid Can Be Decreased By.
From sciencenotes.org
Boiling Point Definition, Temperature, and Examples The Boiling Point Of Liquid Can Be Decreased By Chemists often purify liquids by boiling them and collecting the vapor, a process known as distillation. In an open system this is called atmospheric pressure. The boiling point is the temperature at which the vapor pressure of a liquid is equal to the external pressure. Some liquids have such high normal boiling points that they begin to decompose before distillation. The Boiling Point Of Liquid Can Be Decreased By.
From www.chegg.com
Solved The normal boiling point of liquid bromoethane is The Boiling Point Of Liquid Can Be Decreased By Less than one atmosphere, the boiling point of the liquid is lower than its normal boiling point. As the altitude increases, the boiling. Chemists often purify liquids by boiling them and collecting the vapor, a process known as distillation. Lowering the surrounding pressure, either by increasing altitude or. Boiling point of a liquid can be increased by increasing the external. The Boiling Point Of Liquid Can Be Decreased By.
From www.numerade.com
SOLVED assertion A two liquids A and B having differences in their The Boiling Point Of Liquid Can Be Decreased By Chemists often purify liquids by boiling them and collecting the vapor, a process known as distillation. In an open system this is called atmospheric pressure. Using a closed system to artificially increase that pressure will raise the boiling point of a liquid. As the altitude increases, the boiling. Some liquids have such high normal boiling points that they begin to. The Boiling Point Of Liquid Can Be Decreased By.
From www.chegg.com
Solved The boiling point of a liquid is the temperature at The Boiling Point Of Liquid Can Be Decreased By Some liquids have such high normal boiling points that they begin to decompose before distillation can be carried out. As the altitude increases, the boiling point decreases. Less than one atmosphere, the boiling point of the liquid is lower than its normal boiling point. Boiling point of a liquid can be increased by increasing the external pressure on the liquid,. The Boiling Point Of Liquid Can Be Decreased By.
From www.peoi.org
Chapter 10 Section C Properties of Liquids The Boiling Point Of Liquid Can Be Decreased By Lowering the surrounding pressure, either by increasing altitude or. The boiling point is the temperature at which the vapor pressure of a liquid is equal to the external pressure. As the altitude increases, the boiling. Chemists often purify liquids by boiling them and collecting the vapor, a process known as distillation. Equal to one atmosphere, the boiling point of a. The Boiling Point Of Liquid Can Be Decreased By.
From www.youtube.com
What is boiling point what is the boiling point Factors affecting The Boiling Point Of Liquid Can Be Decreased By Equal to one atmosphere, the boiling point of a liquid is called the normal boiling point. The boiling point is the temperature at which the vapor pressure of a liquid is equal to the external pressure. In an open system this is called atmospheric pressure. Chemists often purify liquids by boiling them and collecting the vapor, a process known as. The Boiling Point Of Liquid Can Be Decreased By.
From www.chegg.com
Solved The boiling point of liquid B is 95.5∘C at 1 atm ( The Boiling Point Of Liquid Can Be Decreased By The boiling point is the temperature at which the vapor pressure of a liquid is equal to the external pressure. The pressure of gas above a liquid affects the boiling point. Lowering the surrounding pressure, either by increasing altitude or. As the altitude increases, the boiling point decreases. Less than one atmosphere, the boiling point of the liquid is lower. The Boiling Point Of Liquid Can Be Decreased By.
From www.youtube.com
Method To Determine Boiling Point Of A Liquid Basic Principles and The Boiling Point Of Liquid Can Be Decreased By Some liquids have such high normal boiling points that they begin to decompose before distillation can be carried out. The boiling point is the temperature at which the vapor pressure of a liquid is equal to the external pressure. The boiling point is the temperature at which the vapor pressure of a liquid is equal to the external pressure. In. The Boiling Point Of Liquid Can Be Decreased By.
From www.learnatnoon.com
The boiling point of water and alcohol explained Noon Academy The Boiling Point Of Liquid Can Be Decreased By The dependence of the boiling point on the external pressure can often be very useful. The boiling point is the temperature at which the vapor pressure of a liquid is equal to the external pressure. In an open system this is called atmospheric pressure. Less than one atmosphere, the boiling point of the liquid is lower than its normal boiling. The Boiling Point Of Liquid Can Be Decreased By.
From www.chegg.com
Solved The boiling point of liquid E is 30.5∘C at 1 atm The Boiling Point Of Liquid Can Be Decreased By Chemists often purify liquids by boiling them and collecting the vapor, a process known as distillation. Lowering the surrounding pressure, either by increasing altitude or. Some liquids have such high normal boiling points that they begin to decompose before distillation can be carried out. As the altitude increases, the boiling point decreases. The pressure of gas above a liquid affects. The Boiling Point Of Liquid Can Be Decreased By.
From slideplayer.com
Intramolecular and Intermolecular Forces ppt download The Boiling Point Of Liquid Can Be Decreased By The pressure of gas above a liquid affects the boiling point. Equal to one atmosphere, the boiling point of a liquid is called the normal boiling point. As the altitude increases, the boiling point decreases. The boiling point is the temperature at which the vapor pressure of a liquid is equal to the external pressure. As the altitude increases, the. The Boiling Point Of Liquid Can Be Decreased By.
From klaubgmaz.blob.core.windows.net
What Is Boiling Point In Science at Lan Johnson blog The Boiling Point Of Liquid Can Be Decreased By As the altitude increases, the boiling. Less than one atmosphere, the boiling point of the liquid is lower than its normal boiling point. The boiling point is the temperature at which the vapor pressure of a liquid is equal to the external pressure. Boiling point of a liquid can be increased by increasing the external pressure on the liquid, whereas. The Boiling Point Of Liquid Can Be Decreased By.
From www.youtube.com
Boiling Points of Liquids, Chemistry Lecture Sabaq.pk YouTube The Boiling Point Of Liquid Can Be Decreased By Lowering the surrounding pressure, either by increasing altitude or. Boiling point of a liquid can be increased by increasing the external pressure on the liquid, whereas it can be decreased by decreasing the. Some liquids have such high normal boiling points that they begin to decompose before distillation can be carried out. Using a closed system to artificially increase that. The Boiling Point Of Liquid Can Be Decreased By.
From slideplayer.com
Physical and Chemical Properties ppt video online download The Boiling Point Of Liquid Can Be Decreased By Chemists often purify liquids by boiling them and collecting the vapor, a process known as distillation. The boiling point is the temperature at which the vapor pressure of a liquid is equal to the external pressure. Less than one atmosphere, the boiling point of the liquid is lower than its normal boiling point. Boiling point of a liquid can be. The Boiling Point Of Liquid Can Be Decreased By.
From general.chemistrysteps.com
Boiling Point Elevation Chemistry Steps The Boiling Point Of Liquid Can Be Decreased By In an open system this is called atmospheric pressure. Equal to one atmosphere, the boiling point of a liquid is called the normal boiling point. Lowering the surrounding pressure, either by increasing altitude or. Using a closed system to artificially increase that pressure will raise the boiling point of a liquid. Boiling point of a liquid can be increased by. The Boiling Point Of Liquid Can Be Decreased By.
From www.slideserve.com
PPT Ch. 13 States of Matter PowerPoint Presentation, free download The Boiling Point Of Liquid Can Be Decreased By Less than one atmosphere, the boiling point of the liquid is lower than its normal boiling point. The pressure of gas above a liquid affects the boiling point. Using a closed system to artificially increase that pressure will raise the boiling point of a liquid. The dependence of the boiling point on the external pressure can often be very useful.. The Boiling Point Of Liquid Can Be Decreased By.
From www.youtube.com
3 5 The boiling points of liquids can be used to separate mixtures The Boiling Point Of Liquid Can Be Decreased By The pressure of gas above a liquid affects the boiling point. Lowering the surrounding pressure, either by increasing altitude or. Chemists often purify liquids by boiling them and collecting the vapor, a process known as distillation. Boiling point of a liquid can be increased by increasing the external pressure on the liquid, whereas it can be decreased by decreasing the.. The Boiling Point Of Liquid Can Be Decreased By.
From www.physicsfox.org
Melting & Boiling • Matter • Physics Fox The Boiling Point Of Liquid Can Be Decreased By Boiling point of a liquid can be increased by increasing the external pressure on the liquid, whereas it can be decreased by decreasing the. Equal to one atmosphere, the boiling point of a liquid is called the normal boiling point. As the altitude increases, the boiling point decreases. As the altitude increases, the boiling. Less than one atmosphere, the boiling. The Boiling Point Of Liquid Can Be Decreased By.
From www.youtube.com
Determine the Boiling Point of a Given Organic Liquid, Chemistry The Boiling Point Of Liquid Can Be Decreased By The pressure of gas above a liquid affects the boiling point. Some liquids have such high normal boiling points that they begin to decompose before distillation can be carried out. Equal to one atmosphere, the boiling point of a liquid is called the normal boiling point. Chemists often purify liquids by boiling them and collecting the vapor, a process known. The Boiling Point Of Liquid Can Be Decreased By.