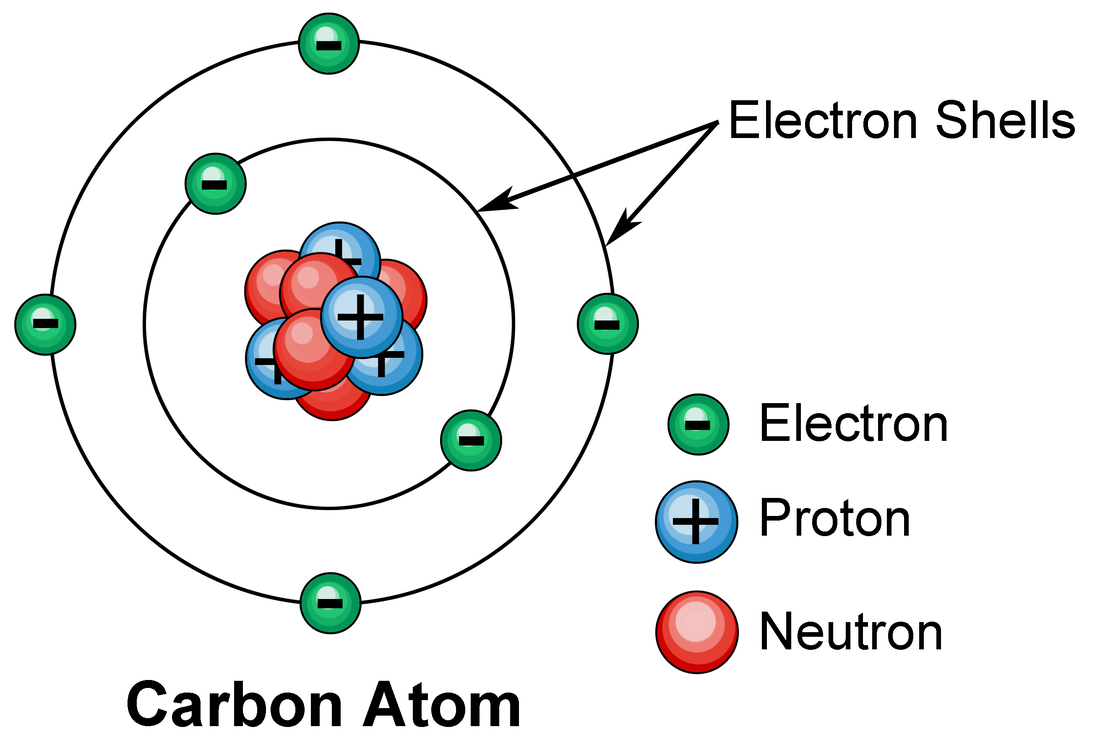
Does Atom Carry Electric Charge . A quantum number that determines the electromagnetic interactions of some subatomic particles; Electrons are said to carry negative charge, while protons are said to. in an electrically neutral atom, the total negative charge of the collection of electrons is equal to the total positive charge in the nucleus. most electric charge is carried by the electrons and protons within an atom. An atom consists of a positively charged nucleus surrounded by negatively charged. Atoms, the fundamental building blocks of all molecules, consist of three types of particles: electric charge, basic property of matter carried by some elementary particles that governs how the particles are affected. atoms, however, were known to be electrically neutral, which means that they carry the same amount of positive and. overview of atomic electrical charges. electric charges in atoms are carried by protons and electrons.
from www.sciencesfp.com
most electric charge is carried by the electrons and protons within an atom. atoms, however, were known to be electrically neutral, which means that they carry the same amount of positive and. Atoms, the fundamental building blocks of all molecules, consist of three types of particles: electric charge, basic property of matter carried by some elementary particles that governs how the particles are affected. An atom consists of a positively charged nucleus surrounded by negatively charged. A quantum number that determines the electromagnetic interactions of some subatomic particles; overview of atomic electrical charges. in an electrically neutral atom, the total negative charge of the collection of electrons is equal to the total positive charge in the nucleus. electric charges in atoms are carried by protons and electrons. Electrons are said to carry negative charge, while protons are said to.
Electronic structure of matter. San Francisco de Paula, Science
Does Atom Carry Electric Charge An atom consists of a positively charged nucleus surrounded by negatively charged. overview of atomic electrical charges. electric charges in atoms are carried by protons and electrons. Atoms, the fundamental building blocks of all molecules, consist of three types of particles: A quantum number that determines the electromagnetic interactions of some subatomic particles; atoms, however, were known to be electrically neutral, which means that they carry the same amount of positive and. most electric charge is carried by the electrons and protons within an atom. Electrons are said to carry negative charge, while protons are said to. in an electrically neutral atom, the total negative charge of the collection of electrons is equal to the total positive charge in the nucleus. electric charge, basic property of matter carried by some elementary particles that governs how the particles are affected. An atom consists of a positively charged nucleus surrounded by negatively charged.
From www.slideserve.com
PPT Chapter 5 Electrical currents PowerPoint Presentation, free Does Atom Carry Electric Charge An atom consists of a positively charged nucleus surrounded by negatively charged. overview of atomic electrical charges. in an electrically neutral atom, the total negative charge of the collection of electrons is equal to the total positive charge in the nucleus. most electric charge is carried by the electrons and protons within an atom. A quantum number. Does Atom Carry Electric Charge.
From learn.sparkfun.com
What is Electricity? SparkFun Learn Does Atom Carry Electric Charge Atoms, the fundamental building blocks of all molecules, consist of three types of particles: Electrons are said to carry negative charge, while protons are said to. electric charge, basic property of matter carried by some elementary particles that governs how the particles are affected. in an electrically neutral atom, the total negative charge of the collection of electrons. Does Atom Carry Electric Charge.
From quizpseudology.z21.web.core.windows.net
How To Find Specific Charge Of Ion Does Atom Carry Electric Charge A quantum number that determines the electromagnetic interactions of some subatomic particles; overview of atomic electrical charges. An atom consists of a positively charged nucleus surrounded by negatively charged. most electric charge is carried by the electrons and protons within an atom. atoms, however, were known to be electrically neutral, which means that they carry the same. Does Atom Carry Electric Charge.
From educationhealey.z13.web.core.windows.net
Atoms That Lose Positively Charged Does Atom Carry Electric Charge An atom consists of a positively charged nucleus surrounded by negatively charged. electric charges in atoms are carried by protons and electrons. Electrons are said to carry negative charge, while protons are said to. A quantum number that determines the electromagnetic interactions of some subatomic particles; atoms, however, were known to be electrically neutral, which means that they. Does Atom Carry Electric Charge.
From www.slideserve.com
PPT Electricity PowerPoint Presentation, free download ID6751678 Does Atom Carry Electric Charge Atoms, the fundamental building blocks of all molecules, consist of three types of particles: electric charges in atoms are carried by protons and electrons. An atom consists of a positively charged nucleus surrounded by negatively charged. most electric charge is carried by the electrons and protons within an atom. in an electrically neutral atom, the total negative. Does Atom Carry Electric Charge.
From open.oregonstate.education
2.1 Elements and Atoms The Building Blocks of Matter Anatomy Does Atom Carry Electric Charge Electrons are said to carry negative charge, while protons are said to. overview of atomic electrical charges. atoms, however, were known to be electrically neutral, which means that they carry the same amount of positive and. Atoms, the fundamental building blocks of all molecules, consist of three types of particles: electric charges in atoms are carried by. Does Atom Carry Electric Charge.
From www.atlearner.com
What is Electric Charge? (1 Electrostatics) Atlearner Learn Science Does Atom Carry Electric Charge electric charges in atoms are carried by protons and electrons. A quantum number that determines the electromagnetic interactions of some subatomic particles; electric charge, basic property of matter carried by some elementary particles that governs how the particles are affected. most electric charge is carried by the electrons and protons within an atom. overview of atomic. Does Atom Carry Electric Charge.
From ar.inspiredpencil.com
Neutron Charge Does Atom Carry Electric Charge A quantum number that determines the electromagnetic interactions of some subatomic particles; overview of atomic electrical charges. An atom consists of a positively charged nucleus surrounded by negatively charged. in an electrically neutral atom, the total negative charge of the collection of electrons is equal to the total positive charge in the nucleus. atoms, however, were known. Does Atom Carry Electric Charge.
From users.highland.edu
Lewis Structures and Covalent Bonding Does Atom Carry Electric Charge electric charge, basic property of matter carried by some elementary particles that governs how the particles are affected. An atom consists of a positively charged nucleus surrounded by negatively charged. electric charges in atoms are carried by protons and electrons. most electric charge is carried by the electrons and protons within an atom. Electrons are said to. Does Atom Carry Electric Charge.
From exohekbra.blob.core.windows.net
Does Neutral Attract Negative at Edward Davison blog Does Atom Carry Electric Charge in an electrically neutral atom, the total negative charge of the collection of electrons is equal to the total positive charge in the nucleus. electric charge, basic property of matter carried by some elementary particles that governs how the particles are affected. electric charges in atoms are carried by protons and electrons. atoms, however, were known. Does Atom Carry Electric Charge.
From frecetovbpschematic.z4.web.core.windows.net
Simple Diagram Of An Atom Does Atom Carry Electric Charge in an electrically neutral atom, the total negative charge of the collection of electrons is equal to the total positive charge in the nucleus. A quantum number that determines the electromagnetic interactions of some subatomic particles; most electric charge is carried by the electrons and protons within an atom. An atom consists of a positively charged nucleus surrounded. Does Atom Carry Electric Charge.
From circuitcoafwaicict8k.z13.web.core.windows.net
Simple Diagram Of An Atom Does Atom Carry Electric Charge Atoms, the fundamental building blocks of all molecules, consist of three types of particles: atoms, however, were known to be electrically neutral, which means that they carry the same amount of positive and. electric charges in atoms are carried by protons and electrons. electric charge, basic property of matter carried by some elementary particles that governs how. Does Atom Carry Electric Charge.
From www.youtube.com
Current Electricity Lecture 1 Physics Intermediate Part 2 Does Atom Carry Electric Charge Electrons are said to carry negative charge, while protons are said to. An atom consists of a positively charged nucleus surrounded by negatively charged. overview of atomic electrical charges. A quantum number that determines the electromagnetic interactions of some subatomic particles; atoms, however, were known to be electrically neutral, which means that they carry the same amount of. Does Atom Carry Electric Charge.
From www.scribd.com
Static Electricity Electric Charge Atoms Does Atom Carry Electric Charge in an electrically neutral atom, the total negative charge of the collection of electrons is equal to the total positive charge in the nucleus. Electrons are said to carry negative charge, while protons are said to. atoms, however, were known to be electrically neutral, which means that they carry the same amount of positive and. A quantum number. Does Atom Carry Electric Charge.
From howtomechatronics.com
What is Electric Charge and How Electricity Works How To Mechatronics Does Atom Carry Electric Charge in an electrically neutral atom, the total negative charge of the collection of electrons is equal to the total positive charge in the nucleus. Electrons are said to carry negative charge, while protons are said to. atoms, however, were known to be electrically neutral, which means that they carry the same amount of positive and. A quantum number. Does Atom Carry Electric Charge.
From www.vrogue.co
What Is Electric Charge And How Electricity Works Ele vrogue.co Does Atom Carry Electric Charge atoms, however, were known to be electrically neutral, which means that they carry the same amount of positive and. A quantum number that determines the electromagnetic interactions of some subatomic particles; Atoms, the fundamental building blocks of all molecules, consist of three types of particles: electric charge, basic property of matter carried by some elementary particles that governs. Does Atom Carry Electric Charge.
From maha22.weebly.com
Diagram of positive,negative atoms SCIENCE Does Atom Carry Electric Charge most electric charge is carried by the electrons and protons within an atom. electric charges in atoms are carried by protons and electrons. overview of atomic electrical charges. An atom consists of a positively charged nucleus surrounded by negatively charged. electric charge, basic property of matter carried by some elementary particles that governs how the particles. Does Atom Carry Electric Charge.
From www.shalom-education.com
Atomic Structure and Ions GCSE Chemistry Revision Does Atom Carry Electric Charge in an electrically neutral atom, the total negative charge of the collection of electrons is equal to the total positive charge in the nucleus. atoms, however, were known to be electrically neutral, which means that they carry the same amount of positive and. overview of atomic electrical charges. Electrons are said to carry negative charge, while protons. Does Atom Carry Electric Charge.
From www.alamy.com
Static electricity for example two blue balloons, structure of Oxygen Does Atom Carry Electric Charge atoms, however, were known to be electrically neutral, which means that they carry the same amount of positive and. in an electrically neutral atom, the total negative charge of the collection of electrons is equal to the total positive charge in the nucleus. Atoms, the fundamental building blocks of all molecules, consist of three types of particles: Electrons. Does Atom Carry Electric Charge.
From thebiologyprimer.com
Atoms & Molecules echapter — The Biology Primer Does Atom Carry Electric Charge Electrons are said to carry negative charge, while protons are said to. electric charge, basic property of matter carried by some elementary particles that governs how the particles are affected. Atoms, the fundamental building blocks of all molecules, consist of three types of particles: most electric charge is carried by the electrons and protons within an atom. . Does Atom Carry Electric Charge.
From periodictable.me
What are the Difference Between Charge and Electron? Does Atom Carry Electric Charge An atom consists of a positively charged nucleus surrounded by negatively charged. A quantum number that determines the electromagnetic interactions of some subatomic particles; Atoms, the fundamental building blocks of all molecules, consist of three types of particles: Electrons are said to carry negative charge, while protons are said to. electric charges in atoms are carried by protons and. Does Atom Carry Electric Charge.
From www.mindomo.com
Energy Mind Map Does Atom Carry Electric Charge A quantum number that determines the electromagnetic interactions of some subatomic particles; electric charges in atoms are carried by protons and electrons. most electric charge is carried by the electrons and protons within an atom. An atom consists of a positively charged nucleus surrounded by negatively charged. atoms, however, were known to be electrically neutral, which means. Does Atom Carry Electric Charge.
From physics.stackexchange.com
electric current If electrons flow everywhere in the wire including Does Atom Carry Electric Charge A quantum number that determines the electromagnetic interactions of some subatomic particles; Atoms, the fundamental building blocks of all molecules, consist of three types of particles: overview of atomic electrical charges. electric charges in atoms are carried by protons and electrons. atoms, however, were known to be electrically neutral, which means that they carry the same amount. Does Atom Carry Electric Charge.
From slideplayer.com
ppt download Does Atom Carry Electric Charge A quantum number that determines the electromagnetic interactions of some subatomic particles; Atoms, the fundamental building blocks of all molecules, consist of three types of particles: in an electrically neutral atom, the total negative charge of the collection of electrons is equal to the total positive charge in the nucleus. An atom consists of a positively charged nucleus surrounded. Does Atom Carry Electric Charge.
From www.nagwa.com
Question Video Explaining Why Atoms Do Not Have a Charge Nagwa Does Atom Carry Electric Charge An atom consists of a positively charged nucleus surrounded by negatively charged. Electrons are said to carry negative charge, while protons are said to. most electric charge is carried by the electrons and protons within an atom. overview of atomic electrical charges. A quantum number that determines the electromagnetic interactions of some subatomic particles; Atoms, the fundamental building. Does Atom Carry Electric Charge.
From www.slideserve.com
PPT Chapter 21 Electric Charge and Electric Fields PowerPoint Does Atom Carry Electric Charge atoms, however, were known to be electrically neutral, which means that they carry the same amount of positive and. most electric charge is carried by the electrons and protons within an atom. electric charges in atoms are carried by protons and electrons. An atom consists of a positively charged nucleus surrounded by negatively charged. Atoms, the fundamental. Does Atom Carry Electric Charge.
From lillian-has-patton.blogspot.com
What Happens When an Electron Is Removed From an Atom LillianhasPatton Does Atom Carry Electric Charge most electric charge is carried by the electrons and protons within an atom. Atoms, the fundamental building blocks of all molecules, consist of three types of particles: A quantum number that determines the electromagnetic interactions of some subatomic particles; Electrons are said to carry negative charge, while protons are said to. in an electrically neutral atom, the total. Does Atom Carry Electric Charge.
From exozsttkf.blob.core.windows.net
What Happens When You Static Electricity at Trevor Youmans blog Does Atom Carry Electric Charge most electric charge is carried by the electrons and protons within an atom. An atom consists of a positively charged nucleus surrounded by negatively charged. A quantum number that determines the electromagnetic interactions of some subatomic particles; Atoms, the fundamental building blocks of all molecules, consist of three types of particles: atoms, however, were known to be electrically. Does Atom Carry Electric Charge.
From engineershub.co.in
What Is Current In Electricity? Engineers Hub Does Atom Carry Electric Charge An atom consists of a positively charged nucleus surrounded by negatively charged. overview of atomic electrical charges. A quantum number that determines the electromagnetic interactions of some subatomic particles; Electrons are said to carry negative charge, while protons are said to. in an electrically neutral atom, the total negative charge of the collection of electrons is equal to. Does Atom Carry Electric Charge.
From mizonuphys.blogspot.com
Nuclear Physics Hmawrhmuhna Atom Does Atom Carry Electric Charge Atoms, the fundamental building blocks of all molecules, consist of three types of particles: most electric charge is carried by the electrons and protons within an atom. overview of atomic electrical charges. atoms, however, were known to be electrically neutral, which means that they carry the same amount of positive and. electric charges in atoms are. Does Atom Carry Electric Charge.
From www.sciencesfp.com
Electronic structure of matter. San Francisco de Paula, Science Does Atom Carry Electric Charge Atoms, the fundamental building blocks of all molecules, consist of three types of particles: A quantum number that determines the electromagnetic interactions of some subatomic particles; electric charges in atoms are carried by protons and electrons. overview of atomic electrical charges. An atom consists of a positively charged nucleus surrounded by negatively charged. Electrons are said to carry. Does Atom Carry Electric Charge.
From enginelibdobchicks.z13.web.core.windows.net
Charges And Electric Forces Diagram Does Atom Carry Electric Charge An atom consists of a positively charged nucleus surrounded by negatively charged. atoms, however, were known to be electrically neutral, which means that they carry the same amount of positive and. A quantum number that determines the electromagnetic interactions of some subatomic particles; Electrons are said to carry negative charge, while protons are said to. in an electrically. Does Atom Carry Electric Charge.
From electric.najura.my.id
An Electrically Charged Atom Is Called Does Atom Carry Electric Charge overview of atomic electrical charges. in an electrically neutral atom, the total negative charge of the collection of electrons is equal to the total positive charge in the nucleus. Atoms, the fundamental building blocks of all molecules, consist of three types of particles: An atom consists of a positively charged nucleus surrounded by negatively charged. most electric. Does Atom Carry Electric Charge.
From socratic.org
What is electronegativity? + Example Does Atom Carry Electric Charge Atoms, the fundamental building blocks of all molecules, consist of three types of particles: in an electrically neutral atom, the total negative charge of the collection of electrons is equal to the total positive charge in the nucleus. A quantum number that determines the electromagnetic interactions of some subatomic particles; Electrons are said to carry negative charge, while protons. Does Atom Carry Electric Charge.
From electricallive.com
Atomic Structure And Electric Charge Electrical engineering interview Does Atom Carry Electric Charge electric charges in atoms are carried by protons and electrons. Atoms, the fundamental building blocks of all molecules, consist of three types of particles: most electric charge is carried by the electrons and protons within an atom. atoms, however, were known to be electrically neutral, which means that they carry the same amount of positive and. Electrons. Does Atom Carry Electric Charge.