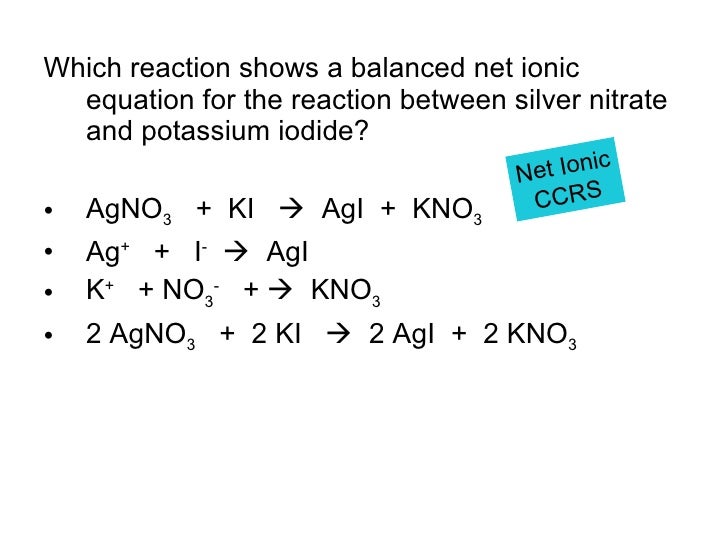
Iron(Ii) Chloride + Silver Nitrate Balanced Equation . the balanced chemical equation for the reaction between iron(ii) chloride and silver nitrate is: The balanced equation for this. how do you write the balanced molecular and net ionic equations for the reaction between aluminum metal and silver nitrate? Fecl 2 (aq) + 2 agno 3 (aq) →. the balanced chemical equation for the reaction between iron(ii) chloride and silver nitrate is: the balanced chemical equation for the reaction between iron (ii) chloride and silver nitrate is: from the balanced equation we use the mol ratio of iron (ii) chloride (fecl 2) to silver nitrate (agno 3). when iron (ii) chloride reacts with silver nitrate, iron (ii) nitrate and silver chloride are produced. It is 1:2, i.e 1. this net ionic equation indicates that solid silver chloride may be produced from dissolved chloride and silver(i) ions,.
from www.slideshare.net
the balanced chemical equation for the reaction between iron(ii) chloride and silver nitrate is: It is 1:2, i.e 1. the balanced chemical equation for the reaction between iron (ii) chloride and silver nitrate is: the balanced chemical equation for the reaction between iron(ii) chloride and silver nitrate is: Fecl 2 (aq) + 2 agno 3 (aq) →. The balanced equation for this. this net ionic equation indicates that solid silver chloride may be produced from dissolved chloride and silver(i) ions,. how do you write the balanced molecular and net ionic equations for the reaction between aluminum metal and silver nitrate? when iron (ii) chloride reacts with silver nitrate, iron (ii) nitrate and silver chloride are produced. from the balanced equation we use the mol ratio of iron (ii) chloride (fecl 2) to silver nitrate (agno 3).
Chemical Reactions
Iron(Ii) Chloride + Silver Nitrate Balanced Equation how do you write the balanced molecular and net ionic equations for the reaction between aluminum metal and silver nitrate? the balanced chemical equation for the reaction between iron (ii) chloride and silver nitrate is: when iron (ii) chloride reacts with silver nitrate, iron (ii) nitrate and silver chloride are produced. how do you write the balanced molecular and net ionic equations for the reaction between aluminum metal and silver nitrate? this net ionic equation indicates that solid silver chloride may be produced from dissolved chloride and silver(i) ions,. the balanced chemical equation for the reaction between iron(ii) chloride and silver nitrate is: the balanced chemical equation for the reaction between iron(ii) chloride and silver nitrate is: from the balanced equation we use the mol ratio of iron (ii) chloride (fecl 2) to silver nitrate (agno 3). Fecl 2 (aq) + 2 agno 3 (aq) →. The balanced equation for this. It is 1:2, i.e 1.
From www.youtube.com
Silver nitrate + sodium chloride YouTube Iron(Ii) Chloride + Silver Nitrate Balanced Equation the balanced chemical equation for the reaction between iron (ii) chloride and silver nitrate is: the balanced chemical equation for the reaction between iron(ii) chloride and silver nitrate is: It is 1:2, i.e 1. the balanced chemical equation for the reaction between iron(ii) chloride and silver nitrate is: from the balanced equation we use the mol. Iron(Ii) Chloride + Silver Nitrate Balanced Equation.
From lena-kcohen.blogspot.com
Net Ionic Equation for Silver Nitrate and Ammonium Carbonate Iron(Ii) Chloride + Silver Nitrate Balanced Equation the balanced chemical equation for the reaction between iron (ii) chloride and silver nitrate is: when iron (ii) chloride reacts with silver nitrate, iron (ii) nitrate and silver chloride are produced. from the balanced equation we use the mol ratio of iron (ii) chloride (fecl 2) to silver nitrate (agno 3). The balanced equation for this. . Iron(Ii) Chloride + Silver Nitrate Balanced Equation.
From www.chegg.com
Solved Silver nitrate reacts with aluminum chloride to form Iron(Ii) Chloride + Silver Nitrate Balanced Equation how do you write the balanced molecular and net ionic equations for the reaction between aluminum metal and silver nitrate? the balanced chemical equation for the reaction between iron(ii) chloride and silver nitrate is: when iron (ii) chloride reacts with silver nitrate, iron (ii) nitrate and silver chloride are produced. Fecl 2 (aq) + 2 agno 3. Iron(Ii) Chloride + Silver Nitrate Balanced Equation.
From www.numerade.com
SOLVED Write balanced equations for each of the following reactions Iron(Ii) Chloride + Silver Nitrate Balanced Equation The balanced equation for this. how do you write the balanced molecular and net ionic equations for the reaction between aluminum metal and silver nitrate? the balanced chemical equation for the reaction between iron(ii) chloride and silver nitrate is: this net ionic equation indicates that solid silver chloride may be produced from dissolved chloride and silver(i) ions,.. Iron(Ii) Chloride + Silver Nitrate Balanced Equation.
From www.slideserve.com
PPT Chapter 4 Chemical Reactions An Introduction PowerPoint Iron(Ii) Chloride + Silver Nitrate Balanced Equation from the balanced equation we use the mol ratio of iron (ii) chloride (fecl 2) to silver nitrate (agno 3). how do you write the balanced molecular and net ionic equations for the reaction between aluminum metal and silver nitrate? when iron (ii) chloride reacts with silver nitrate, iron (ii) nitrate and silver chloride are produced. Fecl. Iron(Ii) Chloride + Silver Nitrate Balanced Equation.
From elchoroukhost.net
Balanced Chemical Equation For Table Salt And Silver Nitrate Elcho Table Iron(Ii) Chloride + Silver Nitrate Balanced Equation from the balanced equation we use the mol ratio of iron (ii) chloride (fecl 2) to silver nitrate (agno 3). the balanced chemical equation for the reaction between iron(ii) chloride and silver nitrate is: the balanced chemical equation for the reaction between iron(ii) chloride and silver nitrate is: how do you write the balanced molecular and. Iron(Ii) Chloride + Silver Nitrate Balanced Equation.
From www.coursehero.com
[Solved] . Suppose 26.0 g of iron(II) chloride is dissolved in 150. mL Iron(Ii) Chloride + Silver Nitrate Balanced Equation the balanced chemical equation for the reaction between iron(ii) chloride and silver nitrate is: The balanced equation for this. the balanced chemical equation for the reaction between iron (ii) chloride and silver nitrate is: how do you write the balanced molecular and net ionic equations for the reaction between aluminum metal and silver nitrate? It is 1:2,. Iron(Ii) Chloride + Silver Nitrate Balanced Equation.
From www.bartleby.com
Answered When aqueous solutions of iron(II)… bartleby Iron(Ii) Chloride + Silver Nitrate Balanced Equation It is 1:2, i.e 1. when iron (ii) chloride reacts with silver nitrate, iron (ii) nitrate and silver chloride are produced. The balanced equation for this. how do you write the balanced molecular and net ionic equations for the reaction between aluminum metal and silver nitrate? this net ionic equation indicates that solid silver chloride may be. Iron(Ii) Chloride + Silver Nitrate Balanced Equation.
From www.numerade.com
SOLVED Aqueous solutions of barium chloride and silver nitrate are Iron(Ii) Chloride + Silver Nitrate Balanced Equation when iron (ii) chloride reacts with silver nitrate, iron (ii) nitrate and silver chloride are produced. Fecl 2 (aq) + 2 agno 3 (aq) →. this net ionic equation indicates that solid silver chloride may be produced from dissolved chloride and silver(i) ions,. the balanced chemical equation for the reaction between iron (ii) chloride and silver nitrate. Iron(Ii) Chloride + Silver Nitrate Balanced Equation.
From www.numerade.com
SOLVED 14. What is the net ionic equation for the reaction of aqueous Iron(Ii) Chloride + Silver Nitrate Balanced Equation the balanced chemical equation for the reaction between iron(ii) chloride and silver nitrate is: this net ionic equation indicates that solid silver chloride may be produced from dissolved chloride and silver(i) ions,. It is 1:2, i.e 1. when iron (ii) chloride reacts with silver nitrate, iron (ii) nitrate and silver chloride are produced. how do you. Iron(Ii) Chloride + Silver Nitrate Balanced Equation.
From www.chegg.com
Solved Silver nitrate and Aluminum chloride react with each Iron(Ii) Chloride + Silver Nitrate Balanced Equation the balanced chemical equation for the reaction between iron (ii) chloride and silver nitrate is: the balanced chemical equation for the reaction between iron(ii) chloride and silver nitrate is: from the balanced equation we use the mol ratio of iron (ii) chloride (fecl 2) to silver nitrate (agno 3). this net ionic equation indicates that solid. Iron(Ii) Chloride + Silver Nitrate Balanced Equation.
From www.chegg.com
Solved a Balance the reaction of sodium hydroxide and Iron(Ii) Chloride + Silver Nitrate Balanced Equation the balanced chemical equation for the reaction between iron (ii) chloride and silver nitrate is: how do you write the balanced molecular and net ionic equations for the reaction between aluminum metal and silver nitrate? Fecl 2 (aq) + 2 agno 3 (aq) →. from the balanced equation we use the mol ratio of iron (ii) chloride. Iron(Ii) Chloride + Silver Nitrate Balanced Equation.
From www.youtube.com
FeCl3+AgNO3=AgCl+Fe(NO3)3 Balanced EquationIron(iii)chloride+Silver Iron(Ii) Chloride + Silver Nitrate Balanced Equation when iron (ii) chloride reacts with silver nitrate, iron (ii) nitrate and silver chloride are produced. Fecl 2 (aq) + 2 agno 3 (aq) →. from the balanced equation we use the mol ratio of iron (ii) chloride (fecl 2) to silver nitrate (agno 3). The balanced equation for this. this net ionic equation indicates that solid. Iron(Ii) Chloride + Silver Nitrate Balanced Equation.
From www.toppr.com
Identify the type of reactions taking place in each of the following Iron(Ii) Chloride + Silver Nitrate Balanced Equation when iron (ii) chloride reacts with silver nitrate, iron (ii) nitrate and silver chloride are produced. the balanced chemical equation for the reaction between iron (ii) chloride and silver nitrate is: The balanced equation for this. It is 1:2, i.e 1. this net ionic equation indicates that solid silver chloride may be produced from dissolved chloride and. Iron(Ii) Chloride + Silver Nitrate Balanced Equation.
From www.numerade.com
SOLVED Net ionic equation Complete iron(III) ionic equation chloride Iron(Ii) Chloride + Silver Nitrate Balanced Equation this net ionic equation indicates that solid silver chloride may be produced from dissolved chloride and silver(i) ions,. the balanced chemical equation for the reaction between iron(ii) chloride and silver nitrate is: The balanced equation for this. the balanced chemical equation for the reaction between iron(ii) chloride and silver nitrate is: Fecl 2 (aq) + 2 agno. Iron(Ii) Chloride + Silver Nitrate Balanced Equation.
From www.numerade.com
SOLVED2, When copper (LL) chloride reacts with silver nitrate, copper Iron(Ii) Chloride + Silver Nitrate Balanced Equation when iron (ii) chloride reacts with silver nitrate, iron (ii) nitrate and silver chloride are produced. the balanced chemical equation for the reaction between iron (ii) chloride and silver nitrate is: the balanced chemical equation for the reaction between iron(ii) chloride and silver nitrate is: the balanced chemical equation for the reaction between iron(ii) chloride and. Iron(Ii) Chloride + Silver Nitrate Balanced Equation.
From shotprofessional22.gitlab.io
Beautiful Silver Nitrate And Copper Ionic Equation Edexcel Igcse Maths Iron(Ii) Chloride + Silver Nitrate Balanced Equation this net ionic equation indicates that solid silver chloride may be produced from dissolved chloride and silver(i) ions,. Fecl 2 (aq) + 2 agno 3 (aq) →. how do you write the balanced molecular and net ionic equations for the reaction between aluminum metal and silver nitrate? The balanced equation for this. the balanced chemical equation for. Iron(Ii) Chloride + Silver Nitrate Balanced Equation.
From www.youtube.com
How to Balance Fe + AgNO3 = Fe(NO3)2 + Ag (Iron + Silver nitrate) YouTube Iron(Ii) Chloride + Silver Nitrate Balanced Equation this net ionic equation indicates that solid silver chloride may be produced from dissolved chloride and silver(i) ions,. the balanced chemical equation for the reaction between iron(ii) chloride and silver nitrate is: It is 1:2, i.e 1. Fecl 2 (aq) + 2 agno 3 (aq) →. The balanced equation for this. the balanced chemical equation for the. Iron(Ii) Chloride + Silver Nitrate Balanced Equation.
From www.numerade.com
SOLVED Silver nitrate is often used in the lab to test for the Iron(Ii) Chloride + Silver Nitrate Balanced Equation when iron (ii) chloride reacts with silver nitrate, iron (ii) nitrate and silver chloride are produced. The balanced equation for this. this net ionic equation indicates that solid silver chloride may be produced from dissolved chloride and silver(i) ions,. from the balanced equation we use the mol ratio of iron (ii) chloride (fecl 2) to silver nitrate. Iron(Ii) Chloride + Silver Nitrate Balanced Equation.
From www.numerade.com
SOLVED For the following reaction, 3.43 grams of iron(II) chloride are Iron(Ii) Chloride + Silver Nitrate Balanced Equation when iron (ii) chloride reacts with silver nitrate, iron (ii) nitrate and silver chloride are produced. Fecl 2 (aq) + 2 agno 3 (aq) →. The balanced equation for this. from the balanced equation we use the mol ratio of iron (ii) chloride (fecl 2) to silver nitrate (agno 3). the balanced chemical equation for the reaction. Iron(Ii) Chloride + Silver Nitrate Balanced Equation.
From www.numerade.com
SOLVED Silver can be precipitated from a silver nitrate solution with Iron(Ii) Chloride + Silver Nitrate Balanced Equation from the balanced equation we use the mol ratio of iron (ii) chloride (fecl 2) to silver nitrate (agno 3). when iron (ii) chloride reacts with silver nitrate, iron (ii) nitrate and silver chloride are produced. It is 1:2, i.e 1. this net ionic equation indicates that solid silver chloride may be produced from dissolved chloride and. Iron(Ii) Chloride + Silver Nitrate Balanced Equation.
From askfilo.com
I Reactions and Equations Silver nitrate + Sodium chloride Silver chlor.. Iron(Ii) Chloride + Silver Nitrate Balanced Equation when iron (ii) chloride reacts with silver nitrate, iron (ii) nitrate and silver chloride are produced. the balanced chemical equation for the reaction between iron(ii) chloride and silver nitrate is: the balanced chemical equation for the reaction between iron(ii) chloride and silver nitrate is: It is 1:2, i.e 1. from the balanced equation we use the. Iron(Ii) Chloride + Silver Nitrate Balanced Equation.
From www.teachoo.com
Case Based Class 10 Science The physical states of the reactants Iron(Ii) Chloride + Silver Nitrate Balanced Equation when iron (ii) chloride reacts with silver nitrate, iron (ii) nitrate and silver chloride are produced. It is 1:2, i.e 1. this net ionic equation indicates that solid silver chloride may be produced from dissolved chloride and silver(i) ions,. from the balanced equation we use the mol ratio of iron (ii) chloride (fecl 2) to silver nitrate. Iron(Ii) Chloride + Silver Nitrate Balanced Equation.
From www.chegg.com
Solved a Write molecular equation for the following Iron(Ii) Chloride + Silver Nitrate Balanced Equation from the balanced equation we use the mol ratio of iron (ii) chloride (fecl 2) to silver nitrate (agno 3). It is 1:2, i.e 1. this net ionic equation indicates that solid silver chloride may be produced from dissolved chloride and silver(i) ions,. the balanced chemical equation for the reaction between iron (ii) chloride and silver nitrate. Iron(Ii) Chloride + Silver Nitrate Balanced Equation.
From lena-kcohen.blogspot.com
Net Ionic Equation for Silver Nitrate and Ammonium Carbonate Iron(Ii) Chloride + Silver Nitrate Balanced Equation the balanced chemical equation for the reaction between iron(ii) chloride and silver nitrate is: The balanced equation for this. when iron (ii) chloride reacts with silver nitrate, iron (ii) nitrate and silver chloride are produced. the balanced chemical equation for the reaction between iron (ii) chloride and silver nitrate is: from the balanced equation we use. Iron(Ii) Chloride + Silver Nitrate Balanced Equation.
From exopnayqj.blob.core.windows.net
Silver Nitrate Copper Wire Balanced Equation at Maria Samuels blog Iron(Ii) Chloride + Silver Nitrate Balanced Equation from the balanced equation we use the mol ratio of iron (ii) chloride (fecl 2) to silver nitrate (agno 3). It is 1:2, i.e 1. the balanced chemical equation for the reaction between iron (ii) chloride and silver nitrate is: Fecl 2 (aq) + 2 agno 3 (aq) →. how do you write the balanced molecular and. Iron(Ii) Chloride + Silver Nitrate Balanced Equation.
From www.slideshare.net
Precipitation react2 Iron(Ii) Chloride + Silver Nitrate Balanced Equation from the balanced equation we use the mol ratio of iron (ii) chloride (fecl 2) to silver nitrate (agno 3). Fecl 2 (aq) + 2 agno 3 (aq) →. when iron (ii) chloride reacts with silver nitrate, iron (ii) nitrate and silver chloride are produced. the balanced chemical equation for the reaction between iron(ii) chloride and silver. Iron(Ii) Chloride + Silver Nitrate Balanced Equation.
From exopnayqj.blob.core.windows.net
Silver Nitrate Copper Wire Balanced Equation at Maria Samuels blog Iron(Ii) Chloride + Silver Nitrate Balanced Equation from the balanced equation we use the mol ratio of iron (ii) chloride (fecl 2) to silver nitrate (agno 3). this net ionic equation indicates that solid silver chloride may be produced from dissolved chloride and silver(i) ions,. how do you write the balanced molecular and net ionic equations for the reaction between aluminum metal and silver. Iron(Ii) Chloride + Silver Nitrate Balanced Equation.
From www.numerade.com
SOLVED2, When copper (LL) chloride reacts with silver nitrate, copper Iron(Ii) Chloride + Silver Nitrate Balanced Equation how do you write the balanced molecular and net ionic equations for the reaction between aluminum metal and silver nitrate? The balanced equation for this. the balanced chemical equation for the reaction between iron(ii) chloride and silver nitrate is: Fecl 2 (aq) + 2 agno 3 (aq) →. It is 1:2, i.e 1. when iron (ii) chloride. Iron(Ii) Chloride + Silver Nitrate Balanced Equation.
From exopnayqj.blob.core.windows.net
Silver Nitrate Copper Wire Balanced Equation at Maria Samuels blog Iron(Ii) Chloride + Silver Nitrate Balanced Equation It is 1:2, i.e 1. how do you write the balanced molecular and net ionic equations for the reaction between aluminum metal and silver nitrate? from the balanced equation we use the mol ratio of iron (ii) chloride (fecl 2) to silver nitrate (agno 3). the balanced chemical equation for the reaction between iron (ii) chloride and. Iron(Ii) Chloride + Silver Nitrate Balanced Equation.
From www.numerade.com
A solution of Iron (II) nitrate is poured into a sodium sulfide Iron(Ii) Chloride + Silver Nitrate Balanced Equation It is 1:2, i.e 1. this net ionic equation indicates that solid silver chloride may be produced from dissolved chloride and silver(i) ions,. the balanced chemical equation for the reaction between iron(ii) chloride and silver nitrate is: the balanced chemical equation for the reaction between iron(ii) chloride and silver nitrate is: how do you write the. Iron(Ii) Chloride + Silver Nitrate Balanced Equation.
From www.numerade.com
SOLVED Write the balanced equation for the reaction of chloride and Iron(Ii) Chloride + Silver Nitrate Balanced Equation The balanced equation for this. from the balanced equation we use the mol ratio of iron (ii) chloride (fecl 2) to silver nitrate (agno 3). It is 1:2, i.e 1. the balanced chemical equation for the reaction between iron(ii) chloride and silver nitrate is: the balanced chemical equation for the reaction between iron (ii) chloride and silver. Iron(Ii) Chloride + Silver Nitrate Balanced Equation.
From angelo-bogspotharrison.blogspot.com
Explain the Difference Between Iron Ii Nitrate and Iron Iii Iron(Ii) Chloride + Silver Nitrate Balanced Equation from the balanced equation we use the mol ratio of iron (ii) chloride (fecl 2) to silver nitrate (agno 3). the balanced chemical equation for the reaction between iron(ii) chloride and silver nitrate is: Fecl 2 (aq) + 2 agno 3 (aq) →. The balanced equation for this. when iron (ii) chloride reacts with silver nitrate, iron. Iron(Ii) Chloride + Silver Nitrate Balanced Equation.
From www.slideshare.net
Chemical Reactions Iron(Ii) Chloride + Silver Nitrate Balanced Equation The balanced equation for this. this net ionic equation indicates that solid silver chloride may be produced from dissolved chloride and silver(i) ions,. from the balanced equation we use the mol ratio of iron (ii) chloride (fecl 2) to silver nitrate (agno 3). It is 1:2, i.e 1. when iron (ii) chloride reacts with silver nitrate, iron. Iron(Ii) Chloride + Silver Nitrate Balanced Equation.
From elchoroukhost.net
Balanced Chemical Equation For Table Salt And Silver Nitrate Elcho Table Iron(Ii) Chloride + Silver Nitrate Balanced Equation Fecl 2 (aq) + 2 agno 3 (aq) →. the balanced chemical equation for the reaction between iron (ii) chloride and silver nitrate is: from the balanced equation we use the mol ratio of iron (ii) chloride (fecl 2) to silver nitrate (agno 3). how do you write the balanced molecular and net ionic equations for the. Iron(Ii) Chloride + Silver Nitrate Balanced Equation.