Medical Device Standards Ppt . Explain fda’s role in regulating medical devices. To download this presentation, visit: This document provides an overview of the regulatory process and classification rules for medical devices in the european union according. The basic regulatory requirements that manufacturers of medical devices distributed in the u.s. Accepted practices in ml/ai algorithm design, development, training, and testing that facilitate the quality development and. Define a medical device and review basics about device classification. This document provides an overview and summary of key changes and requirements in the new medical device regulation (eu) 2017/745, which replaces previous directives. Describe five steps to get a.
from fr.slideshare.net
Explain fda’s role in regulating medical devices. This document provides an overview and summary of key changes and requirements in the new medical device regulation (eu) 2017/745, which replaces previous directives. To download this presentation, visit: The basic regulatory requirements that manufacturers of medical devices distributed in the u.s. Describe five steps to get a. Accepted practices in ml/ai algorithm design, development, training, and testing that facilitate the quality development and. This document provides an overview of the regulatory process and classification rules for medical devices in the european union according. Define a medical device and review basics about device classification.
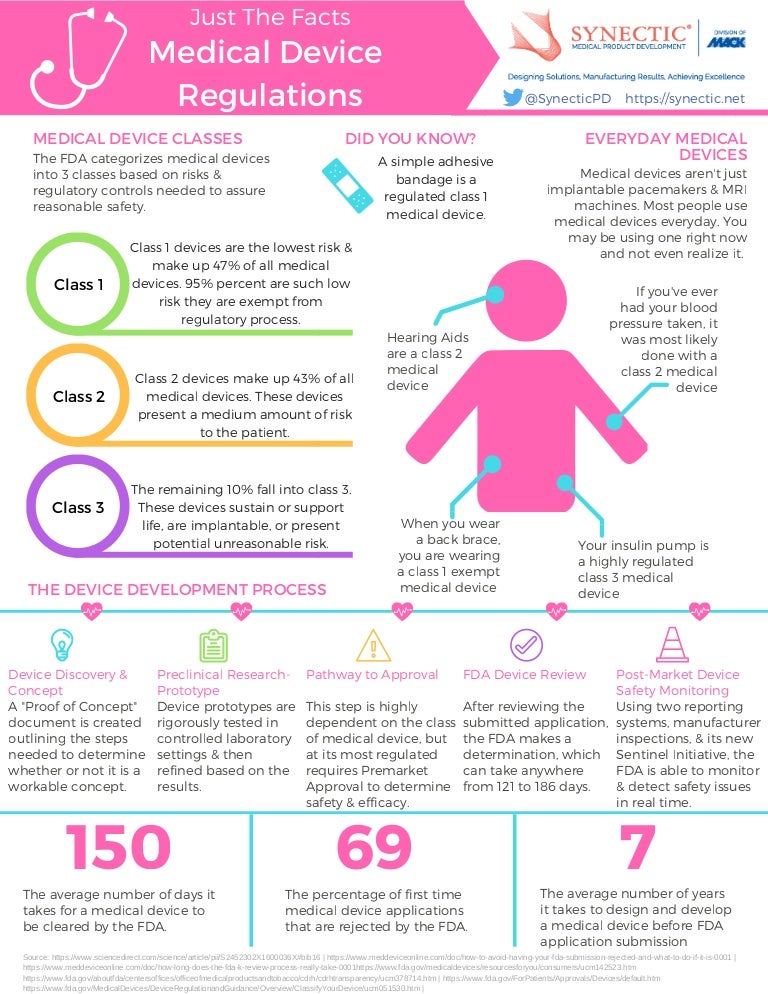
Medical Device FDA Regulations and Classifications infographic
Medical Device Standards Ppt This document provides an overview and summary of key changes and requirements in the new medical device regulation (eu) 2017/745, which replaces previous directives. Explain fda’s role in regulating medical devices. This document provides an overview and summary of key changes and requirements in the new medical device regulation (eu) 2017/745, which replaces previous directives. Define a medical device and review basics about device classification. Accepted practices in ml/ai algorithm design, development, training, and testing that facilitate the quality development and. The basic regulatory requirements that manufacturers of medical devices distributed in the u.s. This document provides an overview of the regulatory process and classification rules for medical devices in the european union according. To download this presentation, visit: Describe five steps to get a.
From www.presentationeze.com
Medical Device Regulations. Design Requirements PresentationEZE Medical Device Standards Ppt To download this presentation, visit: This document provides an overview and summary of key changes and requirements in the new medical device regulation (eu) 2017/745, which replaces previous directives. Accepted practices in ml/ai algorithm design, development, training, and testing that facilitate the quality development and. Define a medical device and review basics about device classification. Describe five steps to get. Medical Device Standards Ppt.
From slidetodoc.com
AAMIs Medical Device Standards Program Presentation to Adva Medical Device Standards Ppt Describe five steps to get a. This document provides an overview and summary of key changes and requirements in the new medical device regulation (eu) 2017/745, which replaces previous directives. Accepted practices in ml/ai algorithm design, development, training, and testing that facilitate the quality development and. The basic regulatory requirements that manufacturers of medical devices distributed in the u.s. To. Medical Device Standards Ppt.
From www.greenlight.guru
Ultimate Guide to ISO 13485 Quality Management System (QMS) for Medical Medical Device Standards Ppt This document provides an overview and summary of key changes and requirements in the new medical device regulation (eu) 2017/745, which replaces previous directives. Accepted practices in ml/ai algorithm design, development, training, and testing that facilitate the quality development and. To download this presentation, visit: Explain fda’s role in regulating medical devices. The basic regulatory requirements that manufacturers of medical. Medical Device Standards Ppt.
From slidetodoc.com
AAMIs Medical Device Standards Program Presentation to Adva Medical Device Standards Ppt This document provides an overview of the regulatory process and classification rules for medical devices in the european union according. This document provides an overview and summary of key changes and requirements in the new medical device regulation (eu) 2017/745, which replaces previous directives. Accepted practices in ml/ai algorithm design, development, training, and testing that facilitate the quality development and.. Medical Device Standards Ppt.
From www.pinterest.com
ISO 134852016 (Medical Devices QMS) Awareness (72slide PowerPoint Medical Device Standards Ppt Describe five steps to get a. This document provides an overview of the regulatory process and classification rules for medical devices in the european union according. Accepted practices in ml/ai algorithm design, development, training, and testing that facilitate the quality development and. To download this presentation, visit: Explain fda’s role in regulating medical devices. Define a medical device and review. Medical Device Standards Ppt.
From slidetodoc.com
AAMIs Medical Device Standards Program Presentation to Adva Medical Device Standards Ppt Define a medical device and review basics about device classification. Accepted practices in ml/ai algorithm design, development, training, and testing that facilitate the quality development and. Describe five steps to get a. This document provides an overview and summary of key changes and requirements in the new medical device regulation (eu) 2017/745, which replaces previous directives. This document provides an. Medical Device Standards Ppt.
From www.slideserve.com
PPT Medical Device Standards PowerPoint Presentation, free download Medical Device Standards Ppt Describe five steps to get a. This document provides an overview of the regulatory process and classification rules for medical devices in the european union according. To download this presentation, visit: Explain fda’s role in regulating medical devices. This document provides an overview and summary of key changes and requirements in the new medical device regulation (eu) 2017/745, which replaces. Medical Device Standards Ppt.
From www.greenlight.guru
Understanding the 5 Phases of Medical Device Development Medical Device Standards Ppt To download this presentation, visit: Define a medical device and review basics about device classification. This document provides an overview and summary of key changes and requirements in the new medical device regulation (eu) 2017/745, which replaces previous directives. This document provides an overview of the regulatory process and classification rules for medical devices in the european union according. Describe. Medical Device Standards Ppt.
From www.slideserve.com
PPT Implantable Medical Devices PowerPoint Presentation, free Medical Device Standards Ppt This document provides an overview of the regulatory process and classification rules for medical devices in the european union according. To download this presentation, visit: Explain fda’s role in regulating medical devices. Describe five steps to get a. Define a medical device and review basics about device classification. The basic regulatory requirements that manufacturers of medical devices distributed in the. Medical Device Standards Ppt.
From www.slideserve.com
PPT IEC TC 62 presentation to 20120626 PowerPoint Medical Device Standards Ppt This document provides an overview of the regulatory process and classification rules for medical devices in the european union according. To download this presentation, visit: Explain fda’s role in regulating medical devices. Accepted practices in ml/ai algorithm design, development, training, and testing that facilitate the quality development and. The basic regulatory requirements that manufacturers of medical devices distributed in the. Medical Device Standards Ppt.
From slidetodoc.com
AAMIs Medical Device Standards Program Presentation to Adva Medical Device Standards Ppt To download this presentation, visit: Accepted practices in ml/ai algorithm design, development, training, and testing that facilitate the quality development and. This document provides an overview of the regulatory process and classification rules for medical devices in the european union according. Describe five steps to get a. The basic regulatory requirements that manufacturers of medical devices distributed in the u.s.. Medical Device Standards Ppt.
From www.youtube.com
Medical Device Usability Highlights of European Regulations and the Medical Device Standards Ppt Describe five steps to get a. To download this presentation, visit: Define a medical device and review basics about device classification. This document provides an overview and summary of key changes and requirements in the new medical device regulation (eu) 2017/745, which replaces previous directives. Accepted practices in ml/ai algorithm design, development, training, and testing that facilitate the quality development. Medical Device Standards Ppt.
From www.slideserve.com
PPT Toward Safe and Effective Wireless Medical Devices and Systems Medical Device Standards Ppt This document provides an overview and summary of key changes and requirements in the new medical device regulation (eu) 2017/745, which replaces previous directives. This document provides an overview of the regulatory process and classification rules for medical devices in the european union according. Describe five steps to get a. To download this presentation, visit: Explain fda’s role in regulating. Medical Device Standards Ppt.
From www.slideserve.com
PPT Regulations of China Medical Device Sunjingsheng Beijing Medical Device Standards Ppt Accepted practices in ml/ai algorithm design, development, training, and testing that facilitate the quality development and. To download this presentation, visit: Explain fda’s role in regulating medical devices. This document provides an overview of the regulatory process and classification rules for medical devices in the european union according. Define a medical device and review basics about device classification. The basic. Medical Device Standards Ppt.
From www.greenlight.guru
ISO Standards for Medical Devices Ultimate List & Overview Medical Device Standards Ppt This document provides an overview of the regulatory process and classification rules for medical devices in the european union according. The basic regulatory requirements that manufacturers of medical devices distributed in the u.s. This document provides an overview and summary of key changes and requirements in the new medical device regulation (eu) 2017/745, which replaces previous directives. Define a medical. Medical Device Standards Ppt.
From www.slideserve.com
PPT Overview of FDA Device Regulations PowerPoint Presentation, free Medical Device Standards Ppt To download this presentation, visit: Describe five steps to get a. Define a medical device and review basics about device classification. This document provides an overview and summary of key changes and requirements in the new medical device regulation (eu) 2017/745, which replaces previous directives. The basic regulatory requirements that manufacturers of medical devices distributed in the u.s. Accepted practices. Medical Device Standards Ppt.
From pubrica.com
A systematic review of quality standards for medical devices and Medical Device Standards Ppt To download this presentation, visit: This document provides an overview of the regulatory process and classification rules for medical devices in the european union according. Explain fda’s role in regulating medical devices. The basic regulatory requirements that manufacturers of medical devices distributed in the u.s. Describe five steps to get a. Accepted practices in ml/ai algorithm design, development, training, and. Medical Device Standards Ppt.
From www.slideteam.net
Testing Medical Devices Ppt Powerpoint Presentation Gallery Format Medical Device Standards Ppt To download this presentation, visit: The basic regulatory requirements that manufacturers of medical devices distributed in the u.s. Define a medical device and review basics about device classification. This document provides an overview of the regulatory process and classification rules for medical devices in the european union according. Explain fda’s role in regulating medical devices. Describe five steps to get. Medical Device Standards Ppt.
From www.slideserve.com
PPT Medical Device Resources PowerPoint Presentation, free download Medical Device Standards Ppt To download this presentation, visit: Define a medical device and review basics about device classification. Accepted practices in ml/ai algorithm design, development, training, and testing that facilitate the quality development and. This document provides an overview and summary of key changes and requirements in the new medical device regulation (eu) 2017/745, which replaces previous directives. Describe five steps to get. Medical Device Standards Ppt.
From slidetodoc.com
AAMIs Medical Device Standards Program Presentation to Adva Medical Device Standards Ppt This document provides an overview and summary of key changes and requirements in the new medical device regulation (eu) 2017/745, which replaces previous directives. The basic regulatory requirements that manufacturers of medical devices distributed in the u.s. Explain fda’s role in regulating medical devices. To download this presentation, visit: Define a medical device and review basics about device classification. Accepted. Medical Device Standards Ppt.
From www.apcerls.com
Safety & Regulatory requirements for Medical Devices APCER Life Sciences Medical Device Standards Ppt This document provides an overview of the regulatory process and classification rules for medical devices in the european union according. Accepted practices in ml/ai algorithm design, development, training, and testing that facilitate the quality development and. Define a medical device and review basics about device classification. Explain fda’s role in regulating medical devices. To download this presentation, visit: This document. Medical Device Standards Ppt.
From www.slideserve.com
PPT 歐盟對醫療器材之管理 PowerPoint Presentation, free download ID3399663 Medical Device Standards Ppt Describe five steps to get a. Explain fda’s role in regulating medical devices. Define a medical device and review basics about device classification. This document provides an overview and summary of key changes and requirements in the new medical device regulation (eu) 2017/745, which replaces previous directives. This document provides an overview of the regulatory process and classification rules for. Medical Device Standards Ppt.
From www.presentationeze.com
Good Manufacturing Practice (GMP’s) for Medical Devices PresentationEZE Medical Device Standards Ppt Explain fda’s role in regulating medical devices. This document provides an overview of the regulatory process and classification rules for medical devices in the european union according. This document provides an overview and summary of key changes and requirements in the new medical device regulation (eu) 2017/745, which replaces previous directives. Define a medical device and review basics about device. Medical Device Standards Ppt.
From slidetodoc.com
AAMIs Medical Device Standards Program Presentation to Adva Medical Device Standards Ppt This document provides an overview of the regulatory process and classification rules for medical devices in the european union according. The basic regulatory requirements that manufacturers of medical devices distributed in the u.s. Define a medical device and review basics about device classification. Explain fda’s role in regulating medical devices. This document provides an overview and summary of key changes. Medical Device Standards Ppt.
From security.cybellum.com
Intro to Medical Device Standards & Regulations Cybellum Medical Device Standards Ppt The basic regulatory requirements that manufacturers of medical devices distributed in the u.s. Describe five steps to get a. Explain fda’s role in regulating medical devices. To download this presentation, visit: This document provides an overview and summary of key changes and requirements in the new medical device regulation (eu) 2017/745, which replaces previous directives. Accepted practices in ml/ai algorithm. Medical Device Standards Ppt.
From slidetodoc.com
AAMIs Medical Device Standards Program Presentation to Adva Medical Device Standards Ppt Describe five steps to get a. This document provides an overview of the regulatory process and classification rules for medical devices in the european union according. This document provides an overview and summary of key changes and requirements in the new medical device regulation (eu) 2017/745, which replaces previous directives. The basic regulatory requirements that manufacturers of medical devices distributed. Medical Device Standards Ppt.
From www.orielstat.com
Medical Device QMS 101 What It Is, Where It’s Required, and Key Medical Device Standards Ppt Explain fda’s role in regulating medical devices. To download this presentation, visit: This document provides an overview of the regulatory process and classification rules for medical devices in the european union according. The basic regulatory requirements that manufacturers of medical devices distributed in the u.s. This document provides an overview and summary of key changes and requirements in the new. Medical Device Standards Ppt.
From fr.slideshare.net
Medical Device FDA Regulations and Classifications infographic Medical Device Standards Ppt To download this presentation, visit: This document provides an overview of the regulatory process and classification rules for medical devices in the european union according. Describe five steps to get a. Explain fda’s role in regulating medical devices. Define a medical device and review basics about device classification. This document provides an overview and summary of key changes and requirements. Medical Device Standards Ppt.
From ossmideast.com
Medical device(QMS)ISO 13485 OSS Middle East Certification Medical Device Standards Ppt To download this presentation, visit: The basic regulatory requirements that manufacturers of medical devices distributed in the u.s. Explain fda’s role in regulating medical devices. Define a medical device and review basics about device classification. Describe five steps to get a. This document provides an overview of the regulatory process and classification rules for medical devices in the european union. Medical Device Standards Ppt.
From www.slideteam.net
Six Months Medical Device Development Roadmap With FDA Regulatory Medical Device Standards Ppt This document provides an overview of the regulatory process and classification rules for medical devices in the european union according. Describe five steps to get a. This document provides an overview and summary of key changes and requirements in the new medical device regulation (eu) 2017/745, which replaces previous directives. Explain fda’s role in regulating medical devices. Accepted practices in. Medical Device Standards Ppt.
From www.slideshare.net
Medical Device Standards StateoftheArt by Sam Lazzara Medical Device Standards Ppt To download this presentation, visit: Explain fda’s role in regulating medical devices. The basic regulatory requirements that manufacturers of medical devices distributed in the u.s. Describe five steps to get a. Define a medical device and review basics about device classification. This document provides an overview of the regulatory process and classification rules for medical devices in the european union. Medical Device Standards Ppt.
From studylib.net
Standards Medical Devices Medical Device Standards Ppt Explain fda’s role in regulating medical devices. Accepted practices in ml/ai algorithm design, development, training, and testing that facilitate the quality development and. This document provides an overview and summary of key changes and requirements in the new medical device regulation (eu) 2017/745, which replaces previous directives. Describe five steps to get a. The basic regulatory requirements that manufacturers of. Medical Device Standards Ppt.
From flevy.com
PPT ISO 134852016 (Medical Devices QMS) Awareness Training (67 Medical Device Standards Ppt This document provides an overview and summary of key changes and requirements in the new medical device regulation (eu) 2017/745, which replaces previous directives. Explain fda’s role in regulating medical devices. Describe five steps to get a. Define a medical device and review basics about device classification. To download this presentation, visit: The basic regulatory requirements that manufacturers of medical. Medical Device Standards Ppt.
From www.scribd.com
Medical Devices Standards PDF Medical Device Clinical Trial Medical Device Standards Ppt Describe five steps to get a. This document provides an overview and summary of key changes and requirements in the new medical device regulation (eu) 2017/745, which replaces previous directives. Explain fda’s role in regulating medical devices. This document provides an overview of the regulatory process and classification rules for medical devices in the european union according. The basic regulatory. Medical Device Standards Ppt.
From www.slideserve.com
PPT Medical Device Labeling PowerPoint Presentation, free download Medical Device Standards Ppt Accepted practices in ml/ai algorithm design, development, training, and testing that facilitate the quality development and. Explain fda’s role in regulating medical devices. The basic regulatory requirements that manufacturers of medical devices distributed in the u.s. To download this presentation, visit: This document provides an overview of the regulatory process and classification rules for medical devices in the european union. Medical Device Standards Ppt.