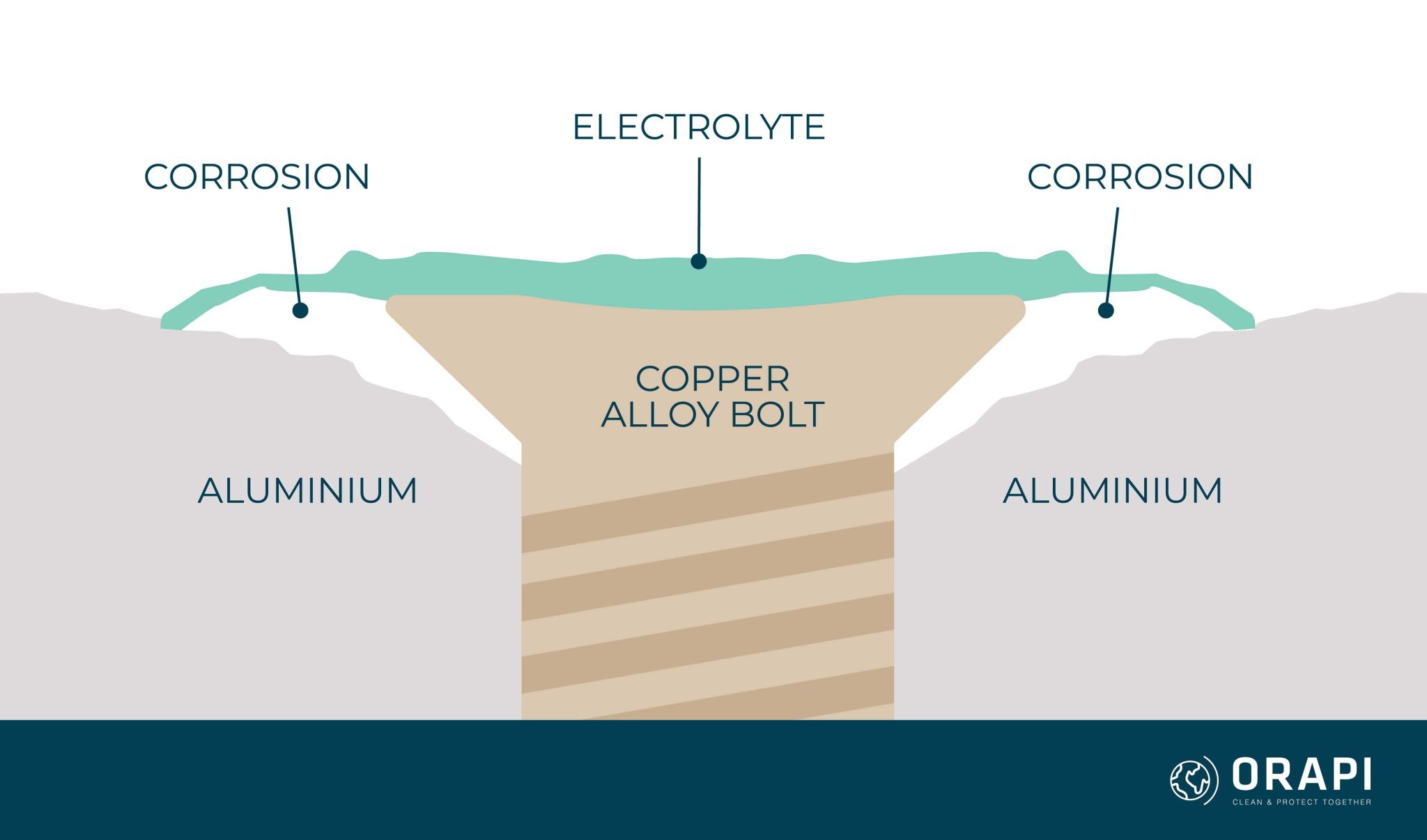
Bronze Brass Galvanic Corrosion . This chart is designed to assist in broadly assessing the risk of galvanic corrosion. Metals close together on the nobility scale corrode less than metals further apart. Brass and bronze go together; Learn how galvanic corrosion occurs when dissimilar metals are in contact with an electrolyte and how to mitigate it with chromium coating solutions. Find out the galvanic series, the area effect, and the common scenarios of galvanic corrosion in marine and plumbing environments. Learn how to avoid galvanic corrosion, a phenomenon that occurs when two metals of different nobility are joined in an electrolytic environment. When the two metals in a galvanic couple are close together on the series, such as manganese bronze and silicon bronze, their voltage ranges. Find a table of anodic index values. Aluminum and zinc make a decent pair; Pair metals with similar voltage differences together. See a galvanic series chart comparing different metals and their electrochemical voltage ranges in seawater, and get tips on choosing the right metals, minimizing cathode area, coating metals, and using inhibitors or sacrificial anodes. Learn about galvanic corrosion, the process by which dissimilar metals oxidize or corrode when in contact. Below is a galvanic reaction chart for dissimilar metals. Like brass, bronze is another copper alloy mixed with tin and small percentages of other elements, but this time, bronze exhibits better. To reduce or prevent galvanic corrosion, consider each of these strategies:
from orapiasia.com
To reduce or prevent galvanic corrosion, consider each of these strategies: Learn how galvanic corrosion occurs when dissimilar metals are in contact with an electrolyte and how to mitigate it with chromium coating solutions. Learn about galvanic corrosion, the process by which dissimilar metals oxidize or corrode when in contact. Find out the galvanic series, the area effect, and the common scenarios of galvanic corrosion in marine and plumbing environments. Learn how to avoid galvanic corrosion, a phenomenon that occurs when two metals of different nobility are joined in an electrolytic environment. Like brass, bronze is another copper alloy mixed with tin and small percentages of other elements, but this time, bronze exhibits better. This chart is designed to assist in broadly assessing the risk of galvanic corrosion. Aluminum and zinc make a decent pair; Pair metals with similar voltage differences together. Below is a galvanic reaction chart for dissimilar metals.
Galvanic Corrosion An InDepth Analysis ORAPI Asia
Bronze Brass Galvanic Corrosion Learn about galvanic corrosion, the process by which dissimilar metals oxidize or corrode when in contact. Aluminum and zinc make a decent pair; Find a table of anodic index values. Learn how to avoid galvanic corrosion, a phenomenon that occurs when two metals of different nobility are joined in an electrolytic environment. Brass and bronze go together; Pair metals with similar voltage differences together. Like brass, bronze is another copper alloy mixed with tin and small percentages of other elements, but this time, bronze exhibits better. Learn about galvanic corrosion, the process by which dissimilar metals oxidize or corrode when in contact. When the two metals in a galvanic couple are close together on the series, such as manganese bronze and silicon bronze, their voltage ranges. Find out the galvanic series, the area effect, and the common scenarios of galvanic corrosion in marine and plumbing environments. Metals close together on the nobility scale corrode less than metals further apart. Below is a galvanic reaction chart for dissimilar metals. See a galvanic series chart comparing different metals and their electrochemical voltage ranges in seawater, and get tips on choosing the right metals, minimizing cathode area, coating metals, and using inhibitors or sacrificial anodes. Learn how galvanic corrosion occurs when dissimilar metals are in contact with an electrolyte and how to mitigate it with chromium coating solutions. To reduce or prevent galvanic corrosion, consider each of these strategies: This chart is designed to assist in broadly assessing the risk of galvanic corrosion.
From www.alideck.co.uk
Galvanic Corrosion » AliDeck Bronze Brass Galvanic Corrosion Below is a galvanic reaction chart for dissimilar metals. See a galvanic series chart comparing different metals and their electrochemical voltage ranges in seawater, and get tips on choosing the right metals, minimizing cathode area, coating metals, and using inhibitors or sacrificial anodes. Aluminum and zinc make a decent pair; Learn how galvanic corrosion occurs when dissimilar metals are in. Bronze Brass Galvanic Corrosion.
From www.intermarineboats.com
Galvanic Corrosion Bronze Brass Galvanic Corrosion Pair metals with similar voltage differences together. This chart is designed to assist in broadly assessing the risk of galvanic corrosion. To reduce or prevent galvanic corrosion, consider each of these strategies: Like brass, bronze is another copper alloy mixed with tin and small percentages of other elements, but this time, bronze exhibits better. Metals close together on the nobility. Bronze Brass Galvanic Corrosion.
From www.simpletwig.com
Galvanic Action Corrosion Prevention Architect's Blog Bronze Brass Galvanic Corrosion Learn about galvanic corrosion, the process by which dissimilar metals oxidize or corrode when in contact. Below is a galvanic reaction chart for dissimilar metals. Find out the galvanic series, the area effect, and the common scenarios of galvanic corrosion in marine and plumbing environments. Pair metals with similar voltage differences together. Find a table of anodic index values. To. Bronze Brass Galvanic Corrosion.
From structx.com
Galvanic Series (electrochemical series) Bronze Brass Galvanic Corrosion See a galvanic series chart comparing different metals and their electrochemical voltage ranges in seawater, and get tips on choosing the right metals, minimizing cathode area, coating metals, and using inhibitors or sacrificial anodes. Learn how galvanic corrosion occurs when dissimilar metals are in contact with an electrolyte and how to mitigate it with chromium coating solutions. Pair metals with. Bronze Brass Galvanic Corrosion.
From www.iccons.com.au
The Insight Galvanic Corrosion and Fasteners ICCONS Bronze Brass Galvanic Corrosion See a galvanic series chart comparing different metals and their electrochemical voltage ranges in seawater, and get tips on choosing the right metals, minimizing cathode area, coating metals, and using inhibitors or sacrificial anodes. Find a table of anodic index values. Find out the galvanic series, the area effect, and the common scenarios of galvanic corrosion in marine and plumbing. Bronze Brass Galvanic Corrosion.
From thailand.swagelok.com
Corrosion Types Swagelok Bronze Brass Galvanic Corrosion Brass and bronze go together; Pair metals with similar voltage differences together. Learn how to avoid galvanic corrosion, a phenomenon that occurs when two metals of different nobility are joined in an electrolytic environment. This chart is designed to assist in broadly assessing the risk of galvanic corrosion. Learn about galvanic corrosion, the process by which dissimilar metals oxidize or. Bronze Brass Galvanic Corrosion.
From forums.mikeholt.com
Replacing copper in service end boxes Page 2 Information by Bronze Brass Galvanic Corrosion This chart is designed to assist in broadly assessing the risk of galvanic corrosion. Pair metals with similar voltage differences together. Learn about galvanic corrosion, the process by which dissimilar metals oxidize or corrode when in contact. See a galvanic series chart comparing different metals and their electrochemical voltage ranges in seawater, and get tips on choosing the right metals,. Bronze Brass Galvanic Corrosion.
From www.cmp-products.com
Galvanic Corrosion CMP Products Limited Bronze Brass Galvanic Corrosion When the two metals in a galvanic couple are close together on the series, such as manganese bronze and silicon bronze, their voltage ranges. To reduce or prevent galvanic corrosion, consider each of these strategies: Find out the galvanic series, the area effect, and the common scenarios of galvanic corrosion in marine and plumbing environments. Find a table of anodic. Bronze Brass Galvanic Corrosion.
From mavink.com
Galvanic Corrosion Chart Dissimilar Metals Bronze Brass Galvanic Corrosion Metals close together on the nobility scale corrode less than metals further apart. When the two metals in a galvanic couple are close together on the series, such as manganese bronze and silicon bronze, their voltage ranges. Learn how galvanic corrosion occurs when dissimilar metals are in contact with an electrolyte and how to mitigate it with chromium coating solutions.. Bronze Brass Galvanic Corrosion.
From www.canada.ca
Understanding galvanic corrosion Canada.ca Bronze Brass Galvanic Corrosion Find a table of anodic index values. Learn how to avoid galvanic corrosion, a phenomenon that occurs when two metals of different nobility are joined in an electrolytic environment. Like brass, bronze is another copper alloy mixed with tin and small percentages of other elements, but this time, bronze exhibits better. Below is a galvanic reaction chart for dissimilar metals.. Bronze Brass Galvanic Corrosion.
From steelfabservices.com.au
Your Guide To Treating Galvanic Corrosion Bronze Brass Galvanic Corrosion Pair metals with similar voltage differences together. Like brass, bronze is another copper alloy mixed with tin and small percentages of other elements, but this time, bronze exhibits better. Aluminum and zinc make a decent pair; Learn how to avoid galvanic corrosion, a phenomenon that occurs when two metals of different nobility are joined in an electrolytic environment. Learn how. Bronze Brass Galvanic Corrosion.
From mungfali.com
Galvanic Corrosion Chart Metals Bronze Brass Galvanic Corrosion See a galvanic series chart comparing different metals and their electrochemical voltage ranges in seawater, and get tips on choosing the right metals, minimizing cathode area, coating metals, and using inhibitors or sacrificial anodes. Pair metals with similar voltage differences together. Aluminum and zinc make a decent pair; Metals close together on the nobility scale corrode less than metals further. Bronze Brass Galvanic Corrosion.
From fairwindfasteners.com
Galvanic Series, or Nobility Chart for Dissimilar Metals Fair Wind Bronze Brass Galvanic Corrosion Aluminum and zinc make a decent pair; Pair metals with similar voltage differences together. Learn how to avoid galvanic corrosion, a phenomenon that occurs when two metals of different nobility are joined in an electrolytic environment. Brass and bronze go together; Find a table of anodic index values. Like brass, bronze is another copper alloy mixed with tin and small. Bronze Brass Galvanic Corrosion.
From www.cmp-products.com
GALVANICCORROSIONTABLE CMP Products Limited Bronze Brass Galvanic Corrosion To reduce or prevent galvanic corrosion, consider each of these strategies: This chart is designed to assist in broadly assessing the risk of galvanic corrosion. Find a table of anodic index values. Find out the galvanic series, the area effect, and the common scenarios of galvanic corrosion in marine and plumbing environments. Pair metals with similar voltage differences together. Learn. Bronze Brass Galvanic Corrosion.
From galvanizeit.org
Dissimilar Metal Corrosion with… American Galvanizers Association Bronze Brass Galvanic Corrosion When the two metals in a galvanic couple are close together on the series, such as manganese bronze and silicon bronze, their voltage ranges. Learn how galvanic corrosion occurs when dissimilar metals are in contact with an electrolyte and how to mitigate it with chromium coating solutions. This chart is designed to assist in broadly assessing the risk of galvanic. Bronze Brass Galvanic Corrosion.
From www.vrogue.co
Galvanic Corrosion A Guide For Architects Galvanic Se vrogue.co Bronze Brass Galvanic Corrosion Pair metals with similar voltage differences together. Learn how galvanic corrosion occurs when dissimilar metals are in contact with an electrolyte and how to mitigate it with chromium coating solutions. Brass and bronze go together; Like brass, bronze is another copper alloy mixed with tin and small percentages of other elements, but this time, bronze exhibits better. Find a table. Bronze Brass Galvanic Corrosion.
From blog.thepipingmart.com
Galvanic Corrosion vs Electrolysis What's the Difference Bronze Brass Galvanic Corrosion Like brass, bronze is another copper alloy mixed with tin and small percentages of other elements, but this time, bronze exhibits better. Find out the galvanic series, the area effect, and the common scenarios of galvanic corrosion in marine and plumbing environments. Pair metals with similar voltage differences together. Aluminum and zinc make a decent pair; Learn how galvanic corrosion. Bronze Brass Galvanic Corrosion.
From www.monarchmetal.com
Galvanic Corrosion Common Questions Answered Bronze Brass Galvanic Corrosion Brass and bronze go together; Learn how galvanic corrosion occurs when dissimilar metals are in contact with an electrolyte and how to mitigate it with chromium coating solutions. When the two metals in a galvanic couple are close together on the series, such as manganese bronze and silicon bronze, their voltage ranges. Learn about galvanic corrosion, the process by which. Bronze Brass Galvanic Corrosion.
From www.youtube.com
How does galvanic corrosion and pitting corrosion occur? (Bimetallic Bronze Brass Galvanic Corrosion Learn how to avoid galvanic corrosion, a phenomenon that occurs when two metals of different nobility are joined in an electrolytic environment. Learn about galvanic corrosion, the process by which dissimilar metals oxidize or corrode when in contact. Like brass, bronze is another copper alloy mixed with tin and small percentages of other elements, but this time, bronze exhibits better.. Bronze Brass Galvanic Corrosion.
From chemistry.stackexchange.com
everyday chemistry Accelerated Oxidation Of Iron When Coating Breaks Bronze Brass Galvanic Corrosion See a galvanic series chart comparing different metals and their electrochemical voltage ranges in seawater, and get tips on choosing the right metals, minimizing cathode area, coating metals, and using inhibitors or sacrificial anodes. Find a table of anodic index values. Metals close together on the nobility scale corrode less than metals further apart. Pair metals with similar voltage differences. Bronze Brass Galvanic Corrosion.
From n-icc.com
Galvanic corrosion وب سایت رسمی شرکت نفتان پایش Corrosion Bronze Brass Galvanic Corrosion Pair metals with similar voltage differences together. Metals close together on the nobility scale corrode less than metals further apart. Learn how to avoid galvanic corrosion, a phenomenon that occurs when two metals of different nobility are joined in an electrolytic environment. This chart is designed to assist in broadly assessing the risk of galvanic corrosion. Like brass, bronze is. Bronze Brass Galvanic Corrosion.
From www.mcfeelys.com
Mixing Metals in Fasteners Bronze Brass Galvanic Corrosion Learn how to avoid galvanic corrosion, a phenomenon that occurs when two metals of different nobility are joined in an electrolytic environment. Below is a galvanic reaction chart for dissimilar metals. Find out the galvanic series, the area effect, and the common scenarios of galvanic corrosion in marine and plumbing environments. See a galvanic series chart comparing different metals and. Bronze Brass Galvanic Corrosion.
From lillymorgan.z13.web.core.windows.net
Dissimilar Metals Corrosion Chart Bronze Brass Galvanic Corrosion Below is a galvanic reaction chart for dissimilar metals. Aluminum and zinc make a decent pair; Like brass, bronze is another copper alloy mixed with tin and small percentages of other elements, but this time, bronze exhibits better. Find a table of anodic index values. Learn about galvanic corrosion, the process by which dissimilar metals oxidize or corrode when in. Bronze Brass Galvanic Corrosion.
From www.scribd.com
Galvanic Corrosion PDF Corrosion Stainless Steel Bronze Brass Galvanic Corrosion Below is a galvanic reaction chart for dissimilar metals. See a galvanic series chart comparing different metals and their electrochemical voltage ranges in seawater, and get tips on choosing the right metals, minimizing cathode area, coating metals, and using inhibitors or sacrificial anodes. Learn how galvanic corrosion occurs when dissimilar metals are in contact with an electrolyte and how to. Bronze Brass Galvanic Corrosion.
From www.wconline.com
What Galvanic Corrosion on Building Facades Can Lead To 20220311 Bronze Brass Galvanic Corrosion Metals close together on the nobility scale corrode less than metals further apart. Below is a galvanic reaction chart for dissimilar metals. Aluminum and zinc make a decent pair; Like brass, bronze is another copper alloy mixed with tin and small percentages of other elements, but this time, bronze exhibits better. Learn how to avoid galvanic corrosion, a phenomenon that. Bronze Brass Galvanic Corrosion.
From mungfali.com
Galvanic Series Chart Bronze Brass Galvanic Corrosion Learn how galvanic corrosion occurs when dissimilar metals are in contact with an electrolyte and how to mitigate it with chromium coating solutions. This chart is designed to assist in broadly assessing the risk of galvanic corrosion. Aluminum and zinc make a decent pair; Pair metals with similar voltage differences together. Learn about galvanic corrosion, the process by which dissimilar. Bronze Brass Galvanic Corrosion.
From blog.thepipingmart.com
Galvanic Corrosion of Brass Steel Bronze Brass Galvanic Corrosion This chart is designed to assist in broadly assessing the risk of galvanic corrosion. Brass and bronze go together; Find out the galvanic series, the area effect, and the common scenarios of galvanic corrosion in marine and plumbing environments. Find a table of anodic index values. When the two metals in a galvanic couple are close together on the series,. Bronze Brass Galvanic Corrosion.
From orapiasia.com
Galvanic Corrosion An InDepth Analysis ORAPI Asia Bronze Brass Galvanic Corrosion Find out the galvanic series, the area effect, and the common scenarios of galvanic corrosion in marine and plumbing environments. Learn how galvanic corrosion occurs when dissimilar metals are in contact with an electrolyte and how to mitigate it with chromium coating solutions. Pair metals with similar voltage differences together. Like brass, bronze is another copper alloy mixed with tin. Bronze Brass Galvanic Corrosion.
From www.icorr.org
Galvanic Corrosion the importance of designingout corrosion hotspot Bronze Brass Galvanic Corrosion Like brass, bronze is another copper alloy mixed with tin and small percentages of other elements, but this time, bronze exhibits better. Find a table of anodic index values. Learn how galvanic corrosion occurs when dissimilar metals are in contact with an electrolyte and how to mitigate it with chromium coating solutions. Learn how to avoid galvanic corrosion, a phenomenon. Bronze Brass Galvanic Corrosion.
From mungfali.com
Galvanic Corrosion Scale Bronze Brass Galvanic Corrosion Learn about galvanic corrosion, the process by which dissimilar metals oxidize or corrode when in contact. Below is a galvanic reaction chart for dissimilar metals. When the two metals in a galvanic couple are close together on the series, such as manganese bronze and silicon bronze, their voltage ranges. See a galvanic series chart comparing different metals and their electrochemical. Bronze Brass Galvanic Corrosion.
From blog.thepipingmart.com
Aluminium bronze vs. Phosphor Bronze Properties Bronze Brass Galvanic Corrosion See a galvanic series chart comparing different metals and their electrochemical voltage ranges in seawater, and get tips on choosing the right metals, minimizing cathode area, coating metals, and using inhibitors or sacrificial anodes. Like brass, bronze is another copper alloy mixed with tin and small percentages of other elements, but this time, bronze exhibits better. Brass and bronze go. Bronze Brass Galvanic Corrosion.
From mungfali.com
Galvanic Corrosion Chart Metals Bronze Brass Galvanic Corrosion This chart is designed to assist in broadly assessing the risk of galvanic corrosion. To reduce or prevent galvanic corrosion, consider each of these strategies: Learn how to avoid galvanic corrosion, a phenomenon that occurs when two metals of different nobility are joined in an electrolytic environment. Find out the galvanic series, the area effect, and the common scenarios of. Bronze Brass Galvanic Corrosion.
From blog.thepipingmart.com
What is Galvanic Corrosion? Bronze Brass Galvanic Corrosion Brass and bronze go together; Pair metals with similar voltage differences together. Learn about galvanic corrosion, the process by which dissimilar metals oxidize or corrode when in contact. Metals close together on the nobility scale corrode less than metals further apart. When the two metals in a galvanic couple are close together on the series, such as manganese bronze and. Bronze Brass Galvanic Corrosion.
From orapiasia.com
Galvanic Corrosion An InDepth Analysis ORAPI Asia Bronze Brass Galvanic Corrosion Aluminum and zinc make a decent pair; To reduce or prevent galvanic corrosion, consider each of these strategies: Find out the galvanic series, the area effect, and the common scenarios of galvanic corrosion in marine and plumbing environments. Learn how to avoid galvanic corrosion, a phenomenon that occurs when two metals of different nobility are joined in an electrolytic environment.. Bronze Brass Galvanic Corrosion.
From mavink.com
Inconel Galvanic Corrosion Chart Bronze Brass Galvanic Corrosion Like brass, bronze is another copper alloy mixed with tin and small percentages of other elements, but this time, bronze exhibits better. To reduce or prevent galvanic corrosion, consider each of these strategies: When the two metals in a galvanic couple are close together on the series, such as manganese bronze and silicon bronze, their voltage ranges. Aluminum and zinc. Bronze Brass Galvanic Corrosion.