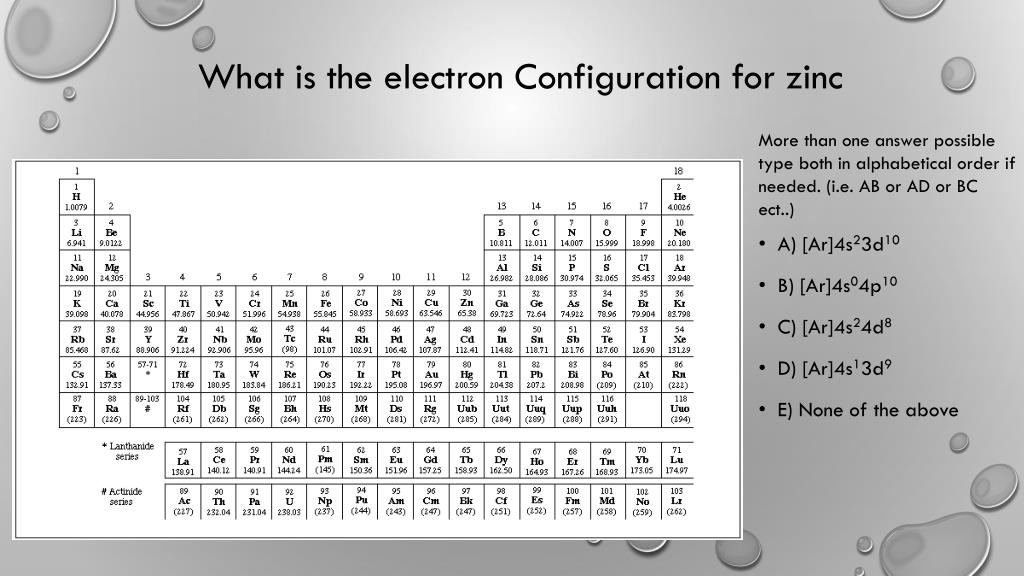
Zinc Electron Configuration Class 10 . The electronic configuration of zinc in the ground state is 1 s 2 2 s 2 2 p 6 3 s 2 3 p 6 3 d 10 4 s 2. #1s^2, 2s^2, 2p^6, 3s^2, 3p^6, 4s^2, 3d^10# Learn how to write electron configuration for all elements using shorthand and full notation. Zinc has two valence electrons in its outer shell. Zinc is a transition metal with atomic number 30 and symbol zn. Electronic configuration of zinc zn: It is used for galvanising, alloys, zinc oxide, and has essential roles in human. See the table with atomic number, element name, shorthand, full configuration. Learn how to write the electronic configuration of zinc (zn) and how to distribute electrons in shells and subshells. [ar] 3d10 4s2 is the electron configuration of zinc. How many valence electrons does zinc have. The zinc atom has 30 protons #=># 30 electrons. Learn how to write the electronic configuration of the first 30 elements with atomic numbers using subshell labels and noble gases. See a table of the electronic configuration of each element and test your understanding with a quiz.
from www.slideserve.com
#1s^2, 2s^2, 2p^6, 3s^2, 3p^6, 4s^2, 3d^10# The zinc atom has 30 protons #=># 30 electrons. Learn how to write electron configuration for all elements using shorthand and full notation. [ar] 3d10 4s2 is the electron configuration of zinc. See the table with atomic number, element name, shorthand, full configuration. The electronic configuration of zinc in the ground state is 1 s 2 2 s 2 2 p 6 3 s 2 3 p 6 3 d 10 4 s 2. Zinc is a transition metal with atomic number 30 and symbol zn. Learn how to write the electronic configuration of the first 30 elements with atomic numbers using subshell labels and noble gases. It is used for galvanising, alloys, zinc oxide, and has essential roles in human. Zinc has two valence electrons in its outer shell.
PPT Electron Configurations PowerPoint Presentation, free download
Zinc Electron Configuration Class 10 Electronic configuration of zinc zn: See a table of the electronic configuration of each element and test your understanding with a quiz. The zinc atom has 30 protons #=># 30 electrons. See the table with atomic number, element name, shorthand, full configuration. Zinc is a transition metal with atomic number 30 and symbol zn. The electronic configuration of zinc in the ground state is 1 s 2 2 s 2 2 p 6 3 s 2 3 p 6 3 d 10 4 s 2. Learn how to write electron configuration for all elements using shorthand and full notation. Learn how to write the electronic configuration of zinc (zn) and how to distribute electrons in shells and subshells. How many valence electrons does zinc have. Zinc has two valence electrons in its outer shell. [ar] 3d10 4s2 is the electron configuration of zinc. It is used for galvanising, alloys, zinc oxide, and has essential roles in human. Learn how to write the electronic configuration of the first 30 elements with atomic numbers using subshell labels and noble gases. #1s^2, 2s^2, 2p^6, 3s^2, 3p^6, 4s^2, 3d^10# Electronic configuration of zinc zn:
From animalia-life.club
Zinc Electron Configuration Zinc Electron Configuration Class 10 The zinc atom has 30 protons #=># 30 electrons. Learn how to write the electronic configuration of the first 30 elements with atomic numbers using subshell labels and noble gases. Learn how to write electron configuration for all elements using shorthand and full notation. [ar] 3d10 4s2 is the electron configuration of zinc. Zinc is a transition metal with atomic. Zinc Electron Configuration Class 10.
From animalia-life.club
Zinc Electron Configuration Zinc Electron Configuration Class 10 Zinc is a transition metal with atomic number 30 and symbol zn. See the table with atomic number, element name, shorthand, full configuration. See a table of the electronic configuration of each element and test your understanding with a quiz. Learn how to write the electronic configuration of the first 30 elements with atomic numbers using subshell labels and noble. Zinc Electron Configuration Class 10.
From animalia-life.club
Zinc Electron Configuration Zinc Electron Configuration Class 10 [ar] 3d10 4s2 is the electron configuration of zinc. Zinc has two valence electrons in its outer shell. Learn how to write the electronic configuration of zinc (zn) and how to distribute electrons in shells and subshells. The zinc atom has 30 protons #=># 30 electrons. See a table of the electronic configuration of each element and test your understanding. Zinc Electron Configuration Class 10.
From animalia-life.club
Zinc Electron Configuration Zinc Electron Configuration Class 10 The zinc atom has 30 protons #=># 30 electrons. See a table of the electronic configuration of each element and test your understanding with a quiz. [ar] 3d10 4s2 is the electron configuration of zinc. Learn how to write the electronic configuration of zinc (zn) and how to distribute electrons in shells and subshells. Zinc has two valence electrons in. Zinc Electron Configuration Class 10.
From ar.inspiredpencil.com
Zinc Electron Configuration Zinc Electron Configuration Class 10 [ar] 3d10 4s2 is the electron configuration of zinc. See the table with atomic number, element name, shorthand, full configuration. How many valence electrons does zinc have. #1s^2, 2s^2, 2p^6, 3s^2, 3p^6, 4s^2, 3d^10# Learn how to write the electronic configuration of the first 30 elements with atomic numbers using subshell labels and noble gases. Learn how to write electron. Zinc Electron Configuration Class 10.
From www.sciencefacts.net
Electron Configuration Definition, Examples, Chart, and Diagram Zinc Electron Configuration Class 10 Learn how to write the electronic configuration of the first 30 elements with atomic numbers using subshell labels and noble gases. How many valence electrons does zinc have. It is used for galvanising, alloys, zinc oxide, and has essential roles in human. Zinc is a transition metal with atomic number 30 and symbol zn. See a table of the electronic. Zinc Electron Configuration Class 10.
From valenceelectrons.com
How Many Valence Electrons Does Zinc (Zn) Have? Zinc Electron Configuration Class 10 #1s^2, 2s^2, 2p^6, 3s^2, 3p^6, 4s^2, 3d^10# It is used for galvanising, alloys, zinc oxide, and has essential roles in human. Learn how to write the electronic configuration of the first 30 elements with atomic numbers using subshell labels and noble gases. Learn how to write electron configuration for all elements using shorthand and full notation. The electronic configuration of. Zinc Electron Configuration Class 10.
From animalia-life.club
Zinc Electron Configuration Zinc Electron Configuration Class 10 Electronic configuration of zinc zn: It is used for galvanising, alloys, zinc oxide, and has essential roles in human. The zinc atom has 30 protons #=># 30 electrons. Learn how to write the electronic configuration of the first 30 elements with atomic numbers using subshell labels and noble gases. Zinc has two valence electrons in its outer shell. See a. Zinc Electron Configuration Class 10.
From www.slideserve.com
PPT Electron Configurations PowerPoint Presentation, free download Zinc Electron Configuration Class 10 The electronic configuration of zinc in the ground state is 1 s 2 2 s 2 2 p 6 3 s 2 3 p 6 3 d 10 4 s 2. Learn how to write electron configuration for all elements using shorthand and full notation. Electronic configuration of zinc zn: See the table with atomic number, element name, shorthand, full. Zinc Electron Configuration Class 10.
From www.youtube.com
zinc electronic configuration How to Write Zinc electronic Zinc Electron Configuration Class 10 Learn how to write the electronic configuration of the first 30 elements with atomic numbers using subshell labels and noble gases. #1s^2, 2s^2, 2p^6, 3s^2, 3p^6, 4s^2, 3d^10# Electronic configuration of zinc zn: See the table with atomic number, element name, shorthand, full configuration. It is used for galvanising, alloys, zinc oxide, and has essential roles in human. How many. Zinc Electron Configuration Class 10.
From animalia-life.club
Zinc Electron Configuration Zinc Electron Configuration Class 10 It is used for galvanising, alloys, zinc oxide, and has essential roles in human. See a table of the electronic configuration of each element and test your understanding with a quiz. Zinc has two valence electrons in its outer shell. The electronic configuration of zinc in the ground state is 1 s 2 2 s 2 2 p 6 3. Zinc Electron Configuration Class 10.
From www.alamy.com
Zinc (Zn). Diagram of the nuclear composition and electron Zinc Electron Configuration Class 10 How many valence electrons does zinc have. Electronic configuration of zinc zn: Learn how to write the electronic configuration of zinc (zn) and how to distribute electrons in shells and subshells. Zinc is a transition metal with atomic number 30 and symbol zn. It is used for galvanising, alloys, zinc oxide, and has essential roles in human. #1s^2, 2s^2, 2p^6,. Zinc Electron Configuration Class 10.
From askfilo.com
Electronic Configuration of Scandium (Z=21) to Zinc (Z=30) In these ele.. Zinc Electron Configuration Class 10 See the table with atomic number, element name, shorthand, full configuration. See a table of the electronic configuration of each element and test your understanding with a quiz. It is used for galvanising, alloys, zinc oxide, and has essential roles in human. The electronic configuration of zinc in the ground state is 1 s 2 2 s 2 2 p. Zinc Electron Configuration Class 10.
From material-properties.org
Zinc Periodic Table and Atomic Properties Zinc Electron Configuration Class 10 See a table of the electronic configuration of each element and test your understanding with a quiz. [ar] 3d10 4s2 is the electron configuration of zinc. Electronic configuration of zinc zn: The zinc atom has 30 protons #=># 30 electrons. The electronic configuration of zinc in the ground state is 1 s 2 2 s 2 2 p 6 3. Zinc Electron Configuration Class 10.
From stock.adobe.com
Zinc Zn Transition metal Chemical Element vector illustration diagram Zinc Electron Configuration Class 10 #1s^2, 2s^2, 2p^6, 3s^2, 3p^6, 4s^2, 3d^10# Zinc is a transition metal with atomic number 30 and symbol zn. The zinc atom has 30 protons #=># 30 electrons. See a table of the electronic configuration of each element and test your understanding with a quiz. Electronic configuration of zinc zn: How many valence electrons does zinc have. Learn how to. Zinc Electron Configuration Class 10.
From www.numerade.com
SOLVED Write the complete electron configuration for the zinc atom Zinc Electron Configuration Class 10 See the table with atomic number, element name, shorthand, full configuration. Zinc has two valence electrons in its outer shell. It is used for galvanising, alloys, zinc oxide, and has essential roles in human. How many valence electrons does zinc have. Learn how to write electron configuration for all elements using shorthand and full notation. The zinc atom has 30. Zinc Electron Configuration Class 10.
From animalia-life.club
Zinc Electron Configuration Zinc Electron Configuration Class 10 The zinc atom has 30 protons #=># 30 electrons. #1s^2, 2s^2, 2p^6, 3s^2, 3p^6, 4s^2, 3d^10# How many valence electrons does zinc have. Learn how to write the electronic configuration of the first 30 elements with atomic numbers using subshell labels and noble gases. Learn how to write the electronic configuration of zinc (zn) and how to distribute electrons in. Zinc Electron Configuration Class 10.
From www.schoolmykids.com
Zinc (Zn) Element Information, Facts, Properties, Uses Periodic Zinc Electron Configuration Class 10 The electronic configuration of zinc in the ground state is 1 s 2 2 s 2 2 p 6 3 s 2 3 p 6 3 d 10 4 s 2. Electronic configuration of zinc zn: See a table of the electronic configuration of each element and test your understanding with a quiz. How many valence electrons does zinc have.. Zinc Electron Configuration Class 10.
From animalia-life.club
Zinc Electron Configuration Zinc Electron Configuration Class 10 Learn how to write electron configuration for all elements using shorthand and full notation. The electronic configuration of zinc in the ground state is 1 s 2 2 s 2 2 p 6 3 s 2 3 p 6 3 d 10 4 s 2. How many valence electrons does zinc have. Learn how to write the electronic configuration of. Zinc Electron Configuration Class 10.
From valenceelectrons.com
How Many Valence Electrons Does Zinc (Zn) Have? Zinc Electron Configuration Class 10 How many valence electrons does zinc have. Learn how to write electron configuration for all elements using shorthand and full notation. The zinc atom has 30 protons #=># 30 electrons. Zinc has two valence electrons in its outer shell. Zinc is a transition metal with atomic number 30 and symbol zn. See the table with atomic number, element name, shorthand,. Zinc Electron Configuration Class 10.
From animalia-life.club
Zinc Electron Configuration Zinc Electron Configuration Class 10 #1s^2, 2s^2, 2p^6, 3s^2, 3p^6, 4s^2, 3d^10# See the table with atomic number, element name, shorthand, full configuration. See a table of the electronic configuration of each element and test your understanding with a quiz. The electronic configuration of zinc in the ground state is 1 s 2 2 s 2 2 p 6 3 s 2 3 p 6. Zinc Electron Configuration Class 10.
From www.britannica.com
zinc Properties, Uses, & Facts Britannica Zinc Electron Configuration Class 10 Zinc has two valence electrons in its outer shell. Learn how to write the electronic configuration of zinc (zn) and how to distribute electrons in shells and subshells. See a table of the electronic configuration of each element and test your understanding with a quiz. How many valence electrons does zinc have. It is used for galvanising, alloys, zinc oxide,. Zinc Electron Configuration Class 10.
From animalia-life.club
Zinc Electron Configuration Zinc Electron Configuration Class 10 How many valence electrons does zinc have. Zinc is a transition metal with atomic number 30 and symbol zn. It is used for galvanising, alloys, zinc oxide, and has essential roles in human. Learn how to write the electronic configuration of zinc (zn) and how to distribute electrons in shells and subshells. See the table with atomic number, element name,. Zinc Electron Configuration Class 10.
From animalia-life.club
Zinc Electron Configuration Zinc Electron Configuration Class 10 Learn how to write electron configuration for all elements using shorthand and full notation. Learn how to write the electronic configuration of zinc (zn) and how to distribute electrons in shells and subshells. See the table with atomic number, element name, shorthand, full configuration. Zinc is a transition metal with atomic number 30 and symbol zn. See a table of. Zinc Electron Configuration Class 10.
From www.numerade.com
SOLVED Write the complete electron configuration for the zinc atom Zinc Electron Configuration Class 10 The zinc atom has 30 protons #=># 30 electrons. Zinc has two valence electrons in its outer shell. Electronic configuration of zinc zn: The electronic configuration of zinc in the ground state is 1 s 2 2 s 2 2 p 6 3 s 2 3 p 6 3 d 10 4 s 2. Learn how to write the electronic. Zinc Electron Configuration Class 10.
From animalia-life.club
Zinc Electron Configuration Zinc Electron Configuration Class 10 It is used for galvanising, alloys, zinc oxide, and has essential roles in human. See the table with atomic number, element name, shorthand, full configuration. The electronic configuration of zinc in the ground state is 1 s 2 2 s 2 2 p 6 3 s 2 3 p 6 3 d 10 4 s 2. Electronic configuration of zinc. Zinc Electron Configuration Class 10.
From animalia-life.club
Zinc Electron Configuration Zinc Electron Configuration Class 10 The electronic configuration of zinc in the ground state is 1 s 2 2 s 2 2 p 6 3 s 2 3 p 6 3 d 10 4 s 2. See a table of the electronic configuration of each element and test your understanding with a quiz. Learn how to write the electronic configuration of the first 30 elements. Zinc Electron Configuration Class 10.
From www.alamy.com
Symbol and electron diagram for Zinc illustration Stock Vector Image Zinc Electron Configuration Class 10 #1s^2, 2s^2, 2p^6, 3s^2, 3p^6, 4s^2, 3d^10# How many valence electrons does zinc have. Zinc is a transition metal with atomic number 30 and symbol zn. Zinc has two valence electrons in its outer shell. See a table of the electronic configuration of each element and test your understanding with a quiz. Learn how to write electron configuration for all. Zinc Electron Configuration Class 10.
From animalia-life.club
Zinc Electron Configuration Zinc Electron Configuration Class 10 It is used for galvanising, alloys, zinc oxide, and has essential roles in human. See the table with atomic number, element name, shorthand, full configuration. The electronic configuration of zinc in the ground state is 1 s 2 2 s 2 2 p 6 3 s 2 3 p 6 3 d 10 4 s 2. Electronic configuration of zinc. Zinc Electron Configuration Class 10.
From chem.libretexts.org
6.9 Electron Configurations and the Periodic Table Chemistry LibreTexts Zinc Electron Configuration Class 10 The zinc atom has 30 protons #=># 30 electrons. Zinc is a transition metal with atomic number 30 and symbol zn. [ar] 3d10 4s2 is the electron configuration of zinc. Zinc has two valence electrons in its outer shell. Learn how to write the electronic configuration of zinc (zn) and how to distribute electrons in shells and subshells. How many. Zinc Electron Configuration Class 10.
From www.youtube.com
Electron Configuration of Zinc Zn Lesson YouTube Zinc Electron Configuration Class 10 How many valence electrons does zinc have. See the table with atomic number, element name, shorthand, full configuration. Learn how to write electron configuration for all elements using shorthand and full notation. The electronic configuration of zinc in the ground state is 1 s 2 2 s 2 2 p 6 3 s 2 3 p 6 3 d 10. Zinc Electron Configuration Class 10.
From www.teachoo.com
[Chemistry Class 12] Account for (c) Zinc is comparatively soft metal Zinc Electron Configuration Class 10 See a table of the electronic configuration of each element and test your understanding with a quiz. Learn how to write the electronic configuration of the first 30 elements with atomic numbers using subshell labels and noble gases. Electronic configuration of zinc zn: Learn how to write the electronic configuration of zinc (zn) and how to distribute electrons in shells. Zinc Electron Configuration Class 10.
From www.alamy.es
Configuración de electrones del zinc. Ilustración de la estructura Zinc Electron Configuration Class 10 The electronic configuration of zinc in the ground state is 1 s 2 2 s 2 2 p 6 3 s 2 3 p 6 3 d 10 4 s 2. Zinc has two valence electrons in its outer shell. Zinc is a transition metal with atomic number 30 and symbol zn. See the table with atomic number, element name,. Zinc Electron Configuration Class 10.
From animalia-life.club
Zinc Electron Configuration Zinc Electron Configuration Class 10 The zinc atom has 30 protons #=># 30 electrons. #1s^2, 2s^2, 2p^6, 3s^2, 3p^6, 4s^2, 3d^10# It is used for galvanising, alloys, zinc oxide, and has essential roles in human. [ar] 3d10 4s2 is the electron configuration of zinc. How many valence electrons does zinc have. Learn how to write the electronic configuration of the first 30 elements with atomic. Zinc Electron Configuration Class 10.
From animalia-life.club
Zinc Electron Configuration Zinc Electron Configuration Class 10 See the table with atomic number, element name, shorthand, full configuration. Zinc has two valence electrons in its outer shell. It is used for galvanising, alloys, zinc oxide, and has essential roles in human. The electronic configuration of zinc in the ground state is 1 s 2 2 s 2 2 p 6 3 s 2 3 p 6 3. Zinc Electron Configuration Class 10.