Differential And Integral Laws . The derivative of a constant is equal to zero. Using calculus, the differential rate law for a chemical reaction can be integrated with respect to time to give an equation that relates. The above is called the definite integral. State the meaning of the fundamental theorem of calculus, part 2. The rate law of a chemical reaction is an equation that links the initial rate with the concentrations (or pressures) of the reactants. ( c ) 0 dx = dx = where ‘c’ is any arbitrary constant. If the region of integration is not specified, the integral is called indefinite. Use the fundamental theorem of calculus, part 1, to evaluate derivatives of integrals. If y = c, dy d. The derivative of the product of a. Rate laws usually include a constant parameter,. Either the differential rate law or the integrated rate law. Then, b f0(x) dx = f(b) − f(a). To apply rate laws to zeroth, first and second order reactions.
from owlcation.com
If y = c, dy d. To apply rate laws to zeroth, first and second order reactions. If the region of integration is not specified, the integral is called indefinite. Either the differential rate law or the integrated rate law. Then, b f0(x) dx = f(b) − f(a). The above is called the definite integral. ( c ) 0 dx = dx = where ‘c’ is any arbitrary constant. Rate laws usually include a constant parameter,. The derivative of a constant is equal to zero. Using calculus, the differential rate law for a chemical reaction can be integrated with respect to time to give an equation that relates.
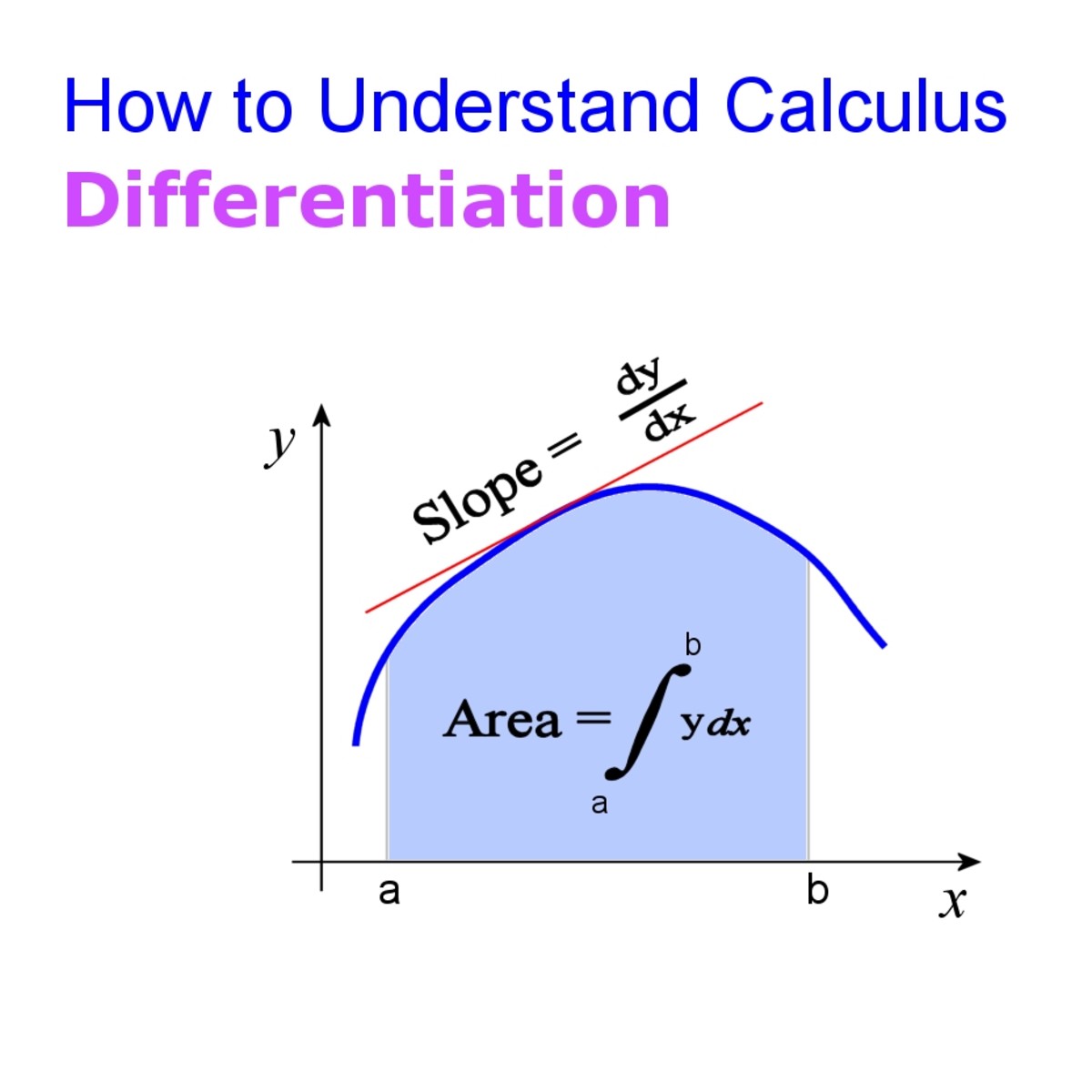
What Is Calculus? A Beginner's Guide to Limits and Differentiation
Differential And Integral Laws Either the differential rate law or the integrated rate law. ( c ) 0 dx = dx = where ‘c’ is any arbitrary constant. Use the fundamental theorem of calculus, part 1, to evaluate derivatives of integrals. Using calculus, the differential rate law for a chemical reaction can be integrated with respect to time to give an equation that relates. Either the differential rate law or the integrated rate law. To apply rate laws to zeroth, first and second order reactions. If y = c, dy d. The derivative of a constant is equal to zero. The rate law of a chemical reaction is an equation that links the initial rate with the concentrations (or pressures) of the reactants. The above is called the definite integral. State the meaning of the fundamental theorem of calculus, part 2. Rate laws usually include a constant parameter,. Then, b f0(x) dx = f(b) − f(a). If the region of integration is not specified, the integral is called indefinite. The derivative of the product of a.
From www.slideserve.com
PPT 5.4 Exponential Functions Differentiation and Integration Differential And Integral Laws State the meaning of the fundamental theorem of calculus, part 2. Use the fundamental theorem of calculus, part 1, to evaluate derivatives of integrals. The derivative of the product of a. Then, b f0(x) dx = f(b) − f(a). The above is called the definite integral. Using calculus, the differential rate law for a chemical reaction can be integrated with. Differential And Integral Laws.
From www.chegg.com
Solved Determine which of the integrals can be found using Differential And Integral Laws ( c ) 0 dx = dx = where ‘c’ is any arbitrary constant. Using calculus, the differential rate law for a chemical reaction can be integrated with respect to time to give an equation that relates. The derivative of a constant is equal to zero. If the region of integration is not specified, the integral is called indefinite. State. Differential And Integral Laws.
From giasutamtaiduc.com
Differentiation and Integration Formula ⭐️⭐️⭐️⭐️⭐ Differential And Integral Laws ( c ) 0 dx = dx = where ‘c’ is any arbitrary constant. Either the differential rate law or the integrated rate law. If y = c, dy d. The rate law of a chemical reaction is an equation that links the initial rate with the concentrations (or pressures) of the reactants. Then, b f0(x) dx = f(b) −. Differential And Integral Laws.
From www.deviantart.com
Derivative and Integral Formula Wallpaper 2 by SawyerTHEBEST on DeviantArt Differential And Integral Laws Then, b f0(x) dx = f(b) − f(a). ( c ) 0 dx = dx = where ‘c’ is any arbitrary constant. The rate law of a chemical reaction is an equation that links the initial rate with the concentrations (or pressures) of the reactants. If y = c, dy d. If the region of integration is not specified, the. Differential And Integral Laws.
From owlcation.com
What Is Calculus? A Beginner's Guide to Limits and Differentiation Differential And Integral Laws The derivative of a constant is equal to zero. Use the fundamental theorem of calculus, part 1, to evaluate derivatives of integrals. Either the differential rate law or the integrated rate law. If y = c, dy d. Using calculus, the differential rate law for a chemical reaction can be integrated with respect to time to give an equation that. Differential And Integral Laws.
From www.studypool.com
SOLUTION Differential and integral calculus Studypool Differential And Integral Laws The above is called the definite integral. State the meaning of the fundamental theorem of calculus, part 2. The derivative of a constant is equal to zero. Either the differential rate law or the integrated rate law. If the region of integration is not specified, the integral is called indefinite. Using calculus, the differential rate law for a chemical reaction. Differential And Integral Laws.
From www.brainkart.com
Basic Rules or Formula of Integration Differential And Integral Laws If y = c, dy d. Use the fundamental theorem of calculus, part 1, to evaluate derivatives of integrals. State the meaning of the fundamental theorem of calculus, part 2. If the region of integration is not specified, the integral is called indefinite. The above is called the definite integral. The derivative of a constant is equal to zero. To. Differential And Integral Laws.
From www.studypool.com
SOLUTION Differential and integral summary of formulas Studypool Differential And Integral Laws Then, b f0(x) dx = f(b) − f(a). The derivative of the product of a. The derivative of a constant is equal to zero. To apply rate laws to zeroth, first and second order reactions. The rate law of a chemical reaction is an equation that links the initial rate with the concentrations (or pressures) of the reactants. The above. Differential And Integral Laws.
From www.tes.com
First order differential equations Teaching Resources Differential And Integral Laws State the meaning of the fundamental theorem of calculus, part 2. If the region of integration is not specified, the integral is called indefinite. The rate law of a chemical reaction is an equation that links the initial rate with the concentrations (or pressures) of the reactants. The derivative of a constant is equal to zero. Rate laws usually include. Differential And Integral Laws.
From www.animalia-life.club
Integration Product Rule Differential And Integral Laws State the meaning of the fundamental theorem of calculus, part 2. The derivative of a constant is equal to zero. The above is called the definite integral. Then, b f0(x) dx = f(b) − f(a). If y = c, dy d. Using calculus, the differential rate law for a chemical reaction can be integrated with respect to time to give. Differential And Integral Laws.
From in.pinterest.com
The Ultimate List of AP® Calculus Tips Albert.io Ap calculus Differential And Integral Laws Then, b f0(x) dx = f(b) − f(a). The above is called the definite integral. The derivative of a constant is equal to zero. Use the fundamental theorem of calculus, part 1, to evaluate derivatives of integrals. ( c ) 0 dx = dx = where ‘c’ is any arbitrary constant. Using calculus, the differential rate law for a chemical. Differential And Integral Laws.
From www.teachmint.com
Integration And Differentiation Formula Maths 11th Notes Teachmint Differential And Integral Laws If the region of integration is not specified, the integral is called indefinite. Then, b f0(x) dx = f(b) − f(a). To apply rate laws to zeroth, first and second order reactions. Use the fundamental theorem of calculus, part 1, to evaluate derivatives of integrals. Rate laws usually include a constant parameter,. The derivative of the product of a. The. Differential And Integral Laws.
From www.docsity.com
Derivative and Integral Cheat Sheet Cheat Sheet Calculus Docsity Differential And Integral Laws Then, b f0(x) dx = f(b) − f(a). Either the differential rate law or the integrated rate law. State the meaning of the fundamental theorem of calculus, part 2. The derivative of the product of a. The derivative of a constant is equal to zero. The rate law of a chemical reaction is an equation that links the initial rate. Differential And Integral Laws.
From www.pleacher.com
Lesson 86 Integration Formulas Differential And Integral Laws Either the differential rate law or the integrated rate law. The derivative of the product of a. If y = c, dy d. Rate laws usually include a constant parameter,. If the region of integration is not specified, the integral is called indefinite. State the meaning of the fundamental theorem of calculus, part 2. The derivative of a constant is. Differential And Integral Laws.
From quizlet.com
AP Calculus AB Derivatives and Integrals Diagram Quizlet Differential And Integral Laws Use the fundamental theorem of calculus, part 1, to evaluate derivatives of integrals. The derivative of a constant is equal to zero. Using calculus, the differential rate law for a chemical reaction can be integrated with respect to time to give an equation that relates. The rate law of a chemical reaction is an equation that links the initial rate. Differential And Integral Laws.
From www.youtube.com
Differential and integral form of gauss law in hindi YouTube Differential And Integral Laws The above is called the definite integral. If y = c, dy d. Using calculus, the differential rate law for a chemical reaction can be integrated with respect to time to give an equation that relates. If the region of integration is not specified, the integral is called indefinite. The derivative of a constant is equal to zero. Use the. Differential And Integral Laws.
From www.slideserve.com
PPT Maxwell’s Equations Differential and Integral Forms PowerPoint Differential And Integral Laws Rate laws usually include a constant parameter,. Using calculus, the differential rate law for a chemical reaction can be integrated with respect to time to give an equation that relates. The above is called the definite integral. State the meaning of the fundamental theorem of calculus, part 2. To apply rate laws to zeroth, first and second order reactions. The. Differential And Integral Laws.
From www.tes.com
A level Maths DIFFERENTIATION Rules (Edexcel) Teaching Resources Differential And Integral Laws The derivative of the product of a. Use the fundamental theorem of calculus, part 1, to evaluate derivatives of integrals. Using calculus, the differential rate law for a chemical reaction can be integrated with respect to time to give an equation that relates. To apply rate laws to zeroth, first and second order reactions. The rate law of a chemical. Differential And Integral Laws.
From physicswood.blogspot.com
Physics Wood BASIC DIFFERENTIATION & INTEGRATION FORMULA Differential And Integral Laws To apply rate laws to zeroth, first and second order reactions. The above is called the definite integral. The derivative of a constant is equal to zero. Using calculus, the differential rate law for a chemical reaction can be integrated with respect to time to give an equation that relates. Use the fundamental theorem of calculus, part 1, to evaluate. Differential And Integral Laws.
From v-fedun.staff.shef.ac.uk
Integration and Differential Equations Differential And Integral Laws State the meaning of the fundamental theorem of calculus, part 2. ( c ) 0 dx = dx = where ‘c’ is any arbitrary constant. The above is called the definite integral. The rate law of a chemical reaction is an equation that links the initial rate with the concentrations (or pressures) of the reactants. To apply rate laws to. Differential And Integral Laws.
From www.theacetutors.com
Derivative Rules Cheat Sheet Calculus Ace Tutors Blog Differential And Integral Laws The above is called the definite integral. State the meaning of the fundamental theorem of calculus, part 2. ( c ) 0 dx = dx = where ‘c’ is any arbitrary constant. Use the fundamental theorem of calculus, part 1, to evaluate derivatives of integrals. Then, b f0(x) dx = f(b) − f(a). Either the differential rate law or the. Differential And Integral Laws.
From dxodbfmjh.blob.core.windows.net
Differential And Integral Method at Terence blog Differential And Integral Laws The derivative of the product of a. If the region of integration is not specified, the integral is called indefinite. Using calculus, the differential rate law for a chemical reaction can be integrated with respect to time to give an equation that relates. Either the differential rate law or the integrated rate law. State the meaning of the fundamental theorem. Differential And Integral Laws.
From www.studypool.com
SOLUTION Differential and integral calculus rules for algebraic Differential And Integral Laws If the region of integration is not specified, the integral is called indefinite. Rate laws usually include a constant parameter,. Use the fundamental theorem of calculus, part 1, to evaluate derivatives of integrals. Either the differential rate law or the integrated rate law. ( c ) 0 dx = dx = where ‘c’ is any arbitrary constant. Then, b f0(x). Differential And Integral Laws.
From www.studypool.com
SOLUTION Differential and integral calculus formula Studypool Differential And Integral Laws State the meaning of the fundamental theorem of calculus, part 2. The derivative of a constant is equal to zero. To apply rate laws to zeroth, first and second order reactions. Use the fundamental theorem of calculus, part 1, to evaluate derivatives of integrals. The derivative of the product of a. The rate law of a chemical reaction is an. Differential And Integral Laws.
From www.nagwa.com
Question Video Finding the Derivative of a Function Defined by an Differential And Integral Laws State the meaning of the fundamental theorem of calculus, part 2. The above is called the definite integral. If the region of integration is not specified, the integral is called indefinite. Then, b f0(x) dx = f(b) − f(a). ( c ) 0 dx = dx = where ‘c’ is any arbitrary constant. If y = c, dy d. The. Differential And Integral Laws.
From ar.inspiredpencil.com
Antiderivative Rules Differential And Integral Laws Either the differential rate law or the integrated rate law. If y = c, dy d. Using calculus, the differential rate law for a chemical reaction can be integrated with respect to time to give an equation that relates. The rate law of a chemical reaction is an equation that links the initial rate with the concentrations (or pressures) of. Differential And Integral Laws.
From nonstopengineering.blogspot.com
Complete Guide for Differentiation and Integration Formulas NonStop Differential And Integral Laws State the meaning of the fundamental theorem of calculus, part 2. If the region of integration is not specified, the integral is called indefinite. Either the differential rate law or the integrated rate law. ( c ) 0 dx = dx = where ‘c’ is any arbitrary constant. Rate laws usually include a constant parameter,. The rate law of a. Differential And Integral Laws.
From reevesapcalcbc.weebly.com
Derivative Rules AP Calc BC Differential And Integral Laws The derivative of a constant is equal to zero. The above is called the definite integral. The derivative of the product of a. To apply rate laws to zeroth, first and second order reactions. Rate laws usually include a constant parameter,. ( c ) 0 dx = dx = where ‘c’ is any arbitrary constant. If the region of integration. Differential And Integral Laws.
From www.youtube.com
6. Rules for finding Particular Integral Case 1 Differential Differential And Integral Laws Rate laws usually include a constant parameter,. The above is called the definite integral. If the region of integration is not specified, the integral is called indefinite. ( c ) 0 dx = dx = where ‘c’ is any arbitrary constant. To apply rate laws to zeroth, first and second order reactions. The derivative of the product of a. The. Differential And Integral Laws.
From www.pinterest.ca
Basic Differential Rules Differential And Integral Laws State the meaning of the fundamental theorem of calculus, part 2. To apply rate laws to zeroth, first and second order reactions. The derivative of a constant is equal to zero. The derivative of the product of a. Either the differential rate law or the integrated rate law. Rate laws usually include a constant parameter,. If y = c, dy. Differential And Integral Laws.
From www.academia.edu
(PDF) Basic Differentiation Rules Basic Integration Formulas Differential And Integral Laws Use the fundamental theorem of calculus, part 1, to evaluate derivatives of integrals. If the region of integration is not specified, the integral is called indefinite. To apply rate laws to zeroth, first and second order reactions. The derivative of the product of a. State the meaning of the fundamental theorem of calculus, part 2. Then, b f0(x) dx =. Differential And Integral Laws.
From in.pinterest.com
Rules Of Differential & Integral Calculus. Math formula chart Differential And Integral Laws Use the fundamental theorem of calculus, part 1, to evaluate derivatives of integrals. Then, b f0(x) dx = f(b) − f(a). The derivative of the product of a. If the region of integration is not specified, the integral is called indefinite. ( c ) 0 dx = dx = where ‘c’ is any arbitrary constant. Using calculus, the differential rate. Differential And Integral Laws.
From www.pinterest.com
Useful derivative and integral formulas Ap calculus, Math formulas Differential And Integral Laws Then, b f0(x) dx = f(b) − f(a). If the region of integration is not specified, the integral is called indefinite. Use the fundamental theorem of calculus, part 1, to evaluate derivatives of integrals. If y = c, dy d. The derivative of the product of a. Rate laws usually include a constant parameter,. To apply rate laws to zeroth,. Differential And Integral Laws.
From www.cuemath.com
All Integration Formulas Complete List of Integrals Cuemath Differential And Integral Laws To apply rate laws to zeroth, first and second order reactions. The rate law of a chemical reaction is an equation that links the initial rate with the concentrations (or pressures) of the reactants. If y = c, dy d. Then, b f0(x) dx = f(b) − f(a). Use the fundamental theorem of calculus, part 1, to evaluate derivatives of. Differential And Integral Laws.
From www.studypool.com
SOLUTION Integration and differentiation formula sheet Studypool Differential And Integral Laws Rate laws usually include a constant parameter,. State the meaning of the fundamental theorem of calculus, part 2. Using calculus, the differential rate law for a chemical reaction can be integrated with respect to time to give an equation that relates. The above is called the definite integral. The derivative of a constant is equal to zero. The rate law. Differential And Integral Laws.