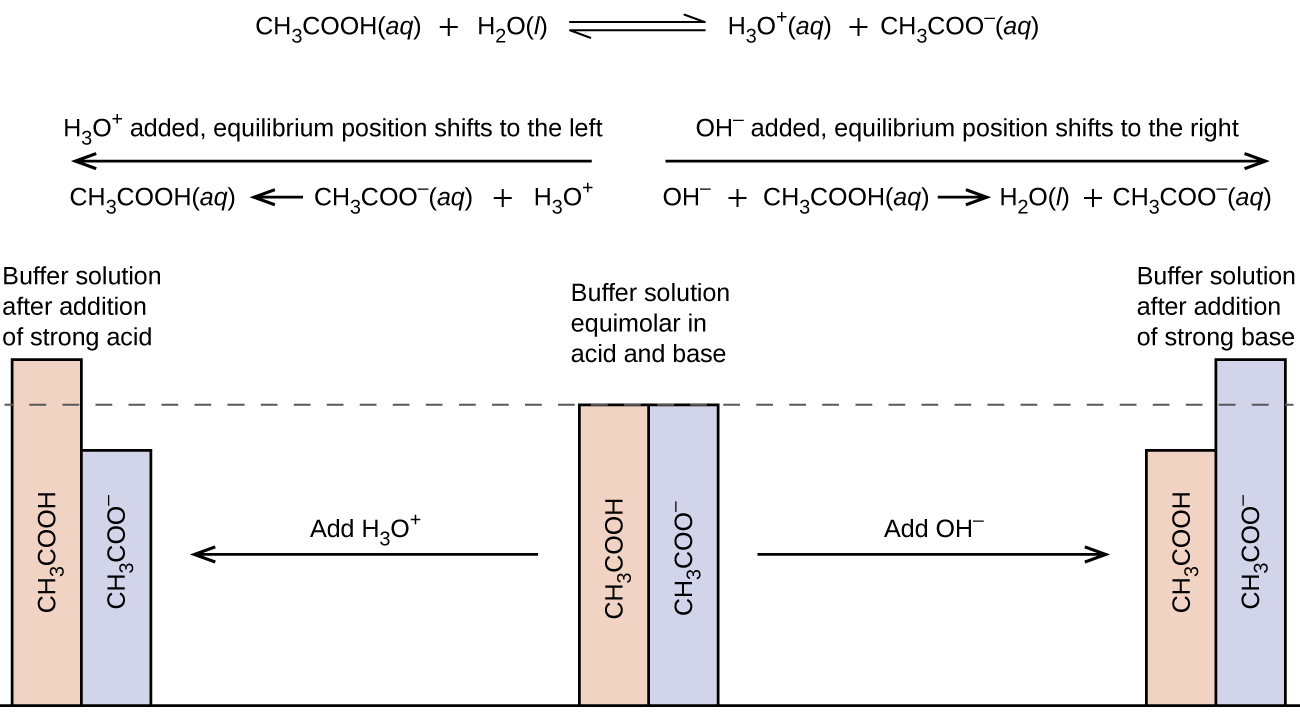
Hcl Added To Buffer . In more rigorous terms, buffer capacity is defined as the number of moles of an acid or base that has to be added to 1 liter of a buffer to cause its ph to change by 1 unit. Adding the hcl (an acid) has changed the ph of the buffer in the acidic direction, from 9.294 to 9.014. As we expect, adding hcl decreases the buffer’s ph by a small amount, dropping from 6.90 to 6.76. 1) i will use 0.500 m hcl for (b). We can use a multiprotic weak acid to prepare buffers at as many different ph’s as there. An ice chart is useful in determining the ph of the system after a strong acid has been added. 50.0 ml of 0.100 m hcl was added to. In a certain $\pu{1 l}$ buffer with $\pu{0.25 m}$ $\ce{nh3}$ and $\pu{0.4 m}$ $\ce{nh4cl}$, $\pu{0.1 mol}$ of $\ce{hcl}$. The standard practice is to assume that $\ce{hcl}$ (being a strong acid) reacts fully with the conjugate base in your buffer solution. Calculate the ph of a buffer before and after the addition of added acid or. The added \(hcl\) (a strong acid) or \(naoh\) (a strong base) will react completely with formate (a weak base) or formic acid (a.
from dandkmotorsports.com
The added \(hcl\) (a strong acid) or \(naoh\) (a strong base) will react completely with formate (a weak base) or formic acid (a. As we expect, adding hcl decreases the buffer’s ph by a small amount, dropping from 6.90 to 6.76. In more rigorous terms, buffer capacity is defined as the number of moles of an acid or base that has to be added to 1 liter of a buffer to cause its ph to change by 1 unit. Adding the hcl (an acid) has changed the ph of the buffer in the acidic direction, from 9.294 to 9.014. The standard practice is to assume that $\ce{hcl}$ (being a strong acid) reacts fully with the conjugate base in your buffer solution. We can use a multiprotic weak acid to prepare buffers at as many different ph’s as there. 1) i will use 0.500 m hcl for (b). In a certain $\pu{1 l}$ buffer with $\pu{0.25 m}$ $\ce{nh3}$ and $\pu{0.4 m}$ $\ce{nh4cl}$, $\pu{0.1 mol}$ of $\ce{hcl}$. Calculate the ph of a buffer before and after the addition of added acid or. An ice chart is useful in determining the ph of the system after a strong acid has been added.
Tris Hcl Buffer Ph 8 5 Recipe Dandk Organizer
Hcl Added To Buffer Calculate the ph of a buffer before and after the addition of added acid or. As we expect, adding hcl decreases the buffer’s ph by a small amount, dropping from 6.90 to 6.76. 1) i will use 0.500 m hcl for (b). Adding the hcl (an acid) has changed the ph of the buffer in the acidic direction, from 9.294 to 9.014. 50.0 ml of 0.100 m hcl was added to. The standard practice is to assume that $\ce{hcl}$ (being a strong acid) reacts fully with the conjugate base in your buffer solution. The added \(hcl\) (a strong acid) or \(naoh\) (a strong base) will react completely with formate (a weak base) or formic acid (a. In more rigorous terms, buffer capacity is defined as the number of moles of an acid or base that has to be added to 1 liter of a buffer to cause its ph to change by 1 unit. In a certain $\pu{1 l}$ buffer with $\pu{0.25 m}$ $\ce{nh3}$ and $\pu{0.4 m}$ $\ce{nh4cl}$, $\pu{0.1 mol}$ of $\ce{hcl}$. An ice chart is useful in determining the ph of the system after a strong acid has been added. Calculate the ph of a buffer before and after the addition of added acid or. We can use a multiprotic weak acid to prepare buffers at as many different ph’s as there.
From askfilo.com
2 Mole of HCl was added to two litre of acid buffer solution. The pH of t.. Hcl Added To Buffer The added \(hcl\) (a strong acid) or \(naoh\) (a strong base) will react completely with formate (a weak base) or formic acid (a. Adding the hcl (an acid) has changed the ph of the buffer in the acidic direction, from 9.294 to 9.014. The standard practice is to assume that $\ce{hcl}$ (being a strong acid) reacts fully with the conjugate. Hcl Added To Buffer.
From chemguru.sg
calculatephofbuffersolution Hcl Added To Buffer Calculate the ph of a buffer before and after the addition of added acid or. 1) i will use 0.500 m hcl for (b). As we expect, adding hcl decreases the buffer’s ph by a small amount, dropping from 6.90 to 6.76. The added \(hcl\) (a strong acid) or \(naoh\) (a strong base) will react completely with formate (a weak. Hcl Added To Buffer.
From schoolbag.info
Titration and Buffers Acids and Bases Training MCAT General Hcl Added To Buffer In more rigorous terms, buffer capacity is defined as the number of moles of an acid or base that has to be added to 1 liter of a buffer to cause its ph to change by 1 unit. 50.0 ml of 0.100 m hcl was added to. The standard practice is to assume that $\ce{hcl}$ (being a strong acid) reacts. Hcl Added To Buffer.
From www.youtube.com
Find the pH of a Buffer after Adding HCl YouTube Hcl Added To Buffer In a certain $\pu{1 l}$ buffer with $\pu{0.25 m}$ $\ce{nh3}$ and $\pu{0.4 m}$ $\ce{nh4cl}$, $\pu{0.1 mol}$ of $\ce{hcl}$. As we expect, adding hcl decreases the buffer’s ph by a small amount, dropping from 6.90 to 6.76. In more rigorous terms, buffer capacity is defined as the number of moles of an acid or base that has to be added to. Hcl Added To Buffer.
From dandkmotorsports.com
Tris Hcl Buffer Ph 8 5 Recipe Dandk Organizer Hcl Added To Buffer As we expect, adding hcl decreases the buffer’s ph by a small amount, dropping from 6.90 to 6.76. An ice chart is useful in determining the ph of the system after a strong acid has been added. 50.0 ml of 0.100 m hcl was added to. The added \(hcl\) (a strong acid) or \(naoh\) (a strong base) will react completely. Hcl Added To Buffer.
From www.youtube.com
Quick video Buffer of NH4/NH3 and addition of HCl to a buffer Hcl Added To Buffer The added \(hcl\) (a strong acid) or \(naoh\) (a strong base) will react completely with formate (a weak base) or formic acid (a. Adding the hcl (an acid) has changed the ph of the buffer in the acidic direction, from 9.294 to 9.014. Calculate the ph of a buffer before and after the addition of added acid or. The standard. Hcl Added To Buffer.
From www.brainkart.com
Buffers Hcl Added To Buffer In a certain $\pu{1 l}$ buffer with $\pu{0.25 m}$ $\ce{nh3}$ and $\pu{0.4 m}$ $\ce{nh4cl}$, $\pu{0.1 mol}$ of $\ce{hcl}$. An ice chart is useful in determining the ph of the system after a strong acid has been added. The standard practice is to assume that $\ce{hcl}$ (being a strong acid) reacts fully with the conjugate base in your buffer solution. 50.0. Hcl Added To Buffer.
From www.numerade.com
SOLVED When hydrochloric acid, HCl , is added dropwise to a buffered Hcl Added To Buffer In a certain $\pu{1 l}$ buffer with $\pu{0.25 m}$ $\ce{nh3}$ and $\pu{0.4 m}$ $\ce{nh4cl}$, $\pu{0.1 mol}$ of $\ce{hcl}$. We can use a multiprotic weak acid to prepare buffers at as many different ph’s as there. 1) i will use 0.500 m hcl for (b). As we expect, adding hcl decreases the buffer’s ph by a small amount, dropping from 6.90. Hcl Added To Buffer.
From www.youtube.com
Calculating pH changes to a buffer solution YouTube Hcl Added To Buffer As we expect, adding hcl decreases the buffer’s ph by a small amount, dropping from 6.90 to 6.76. Calculate the ph of a buffer before and after the addition of added acid or. 1) i will use 0.500 m hcl for (b). The standard practice is to assume that $\ce{hcl}$ (being a strong acid) reacts fully with the conjugate base. Hcl Added To Buffer.
From byjus.com
When a small amount of HCL is added to a buffer solution of acetic acid Hcl Added To Buffer Adding the hcl (an acid) has changed the ph of the buffer in the acidic direction, from 9.294 to 9.014. In more rigorous terms, buffer capacity is defined as the number of moles of an acid or base that has to be added to 1 liter of a buffer to cause its ph to change by 1 unit. Calculate the. Hcl Added To Buffer.
From www.youtube.com
How to calculate the pH of a buffer solution YouTube Hcl Added To Buffer An ice chart is useful in determining the ph of the system after a strong acid has been added. The added \(hcl\) (a strong acid) or \(naoh\) (a strong base) will react completely with formate (a weak base) or formic acid (a. Calculate the ph of a buffer before and after the addition of added acid or. In a certain. Hcl Added To Buffer.
From www.youtube.com
Adding Acid to a Buffer Calculating the pH using Henderson Hcl Added To Buffer Adding the hcl (an acid) has changed the ph of the buffer in the acidic direction, from 9.294 to 9.014. In a certain $\pu{1 l}$ buffer with $\pu{0.25 m}$ $\ce{nh3}$ and $\pu{0.4 m}$ $\ce{nh4cl}$, $\pu{0.1 mol}$ of $\ce{hcl}$. 1) i will use 0.500 m hcl for (b). The added \(hcl\) (a strong acid) or \(naoh\) (a strong base) will react. Hcl Added To Buffer.
From www.youtube.com
Acids and Bases Buffer Calculation Past Paper Exam Question Hcl Added To Buffer The added \(hcl\) (a strong acid) or \(naoh\) (a strong base) will react completely with formate (a weak base) or formic acid (a. 50.0 ml of 0.100 m hcl was added to. The standard practice is to assume that $\ce{hcl}$ (being a strong acid) reacts fully with the conjugate base in your buffer solution. In a certain $\pu{1 l}$ buffer. Hcl Added To Buffer.
From www.slideserve.com
PPT PART 4 Salt Hydrolysis and Buffer Solutions PowerPoint Hcl Added To Buffer 50.0 ml of 0.100 m hcl was added to. We can use a multiprotic weak acid to prepare buffers at as many different ph’s as there. The added \(hcl\) (a strong acid) or \(naoh\) (a strong base) will react completely with formate (a weak base) or formic acid (a. Adding the hcl (an acid) has changed the ph of the. Hcl Added To Buffer.
From saylordotorg.github.io
Buffers Hcl Added To Buffer In more rigorous terms, buffer capacity is defined as the number of moles of an acid or base that has to be added to 1 liter of a buffer to cause its ph to change by 1 unit. As we expect, adding hcl decreases the buffer’s ph by a small amount, dropping from 6.90 to 6.76. We can use a. Hcl Added To Buffer.
From www.chemistrystudent.com
Buffer Solutions (ALevel) ChemistryStudent Hcl Added To Buffer 1) i will use 0.500 m hcl for (b). In a certain $\pu{1 l}$ buffer with $\pu{0.25 m}$ $\ce{nh3}$ and $\pu{0.4 m}$ $\ce{nh4cl}$, $\pu{0.1 mol}$ of $\ce{hcl}$. Calculate the ph of a buffer before and after the addition of added acid or. An ice chart is useful in determining the ph of the system after a strong acid has been. Hcl Added To Buffer.
From www.youtube.com
pH of a buffer after strong acid addition YouTube Hcl Added To Buffer The added \(hcl\) (a strong acid) or \(naoh\) (a strong base) will react completely with formate (a weak base) or formic acid (a. We can use a multiprotic weak acid to prepare buffers at as many different ph’s as there. An ice chart is useful in determining the ph of the system after a strong acid has been added. As. Hcl Added To Buffer.
From www.youtube.com
CALCULATION pH OF BUFFER SOLUTION EXAMPLE 2 YouTube Hcl Added To Buffer An ice chart is useful in determining the ph of the system after a strong acid has been added. 1) i will use 0.500 m hcl for (b). Calculate the ph of a buffer before and after the addition of added acid or. The added \(hcl\) (a strong acid) or \(naoh\) (a strong base) will react completely with formate (a. Hcl Added To Buffer.
From www.peoi.org
Chapter 12 Section G Buffers Hcl Added To Buffer The added \(hcl\) (a strong acid) or \(naoh\) (a strong base) will react completely with formate (a weak base) or formic acid (a. The standard practice is to assume that $\ce{hcl}$ (being a strong acid) reacts fully with the conjugate base in your buffer solution. Calculate the ph of a buffer before and after the addition of added acid or.. Hcl Added To Buffer.
From doctorlib.info
pH and Buffers AcidBase Physiology The Respiratory System Hcl Added To Buffer The added \(hcl\) (a strong acid) or \(naoh\) (a strong base) will react completely with formate (a weak base) or formic acid (a. Calculate the ph of a buffer before and after the addition of added acid or. As we expect, adding hcl decreases the buffer’s ph by a small amount, dropping from 6.90 to 6.76. In more rigorous terms,. Hcl Added To Buffer.
From www.youtube.com
Calculating pH of buffer solution with Strong Acid YouTube Hcl Added To Buffer In more rigorous terms, buffer capacity is defined as the number of moles of an acid or base that has to be added to 1 liter of a buffer to cause its ph to change by 1 unit. The added \(hcl\) (a strong acid) or \(naoh\) (a strong base) will react completely with formate (a weak base) or formic acid. Hcl Added To Buffer.
From classnotes.org.in
Buffer solution and Buffer Action Chemistry, Class 11, Ionic Equilibrium Hcl Added To Buffer The standard practice is to assume that $\ce{hcl}$ (being a strong acid) reacts fully with the conjugate base in your buffer solution. An ice chart is useful in determining the ph of the system after a strong acid has been added. In a certain $\pu{1 l}$ buffer with $\pu{0.25 m}$ $\ce{nh3}$ and $\pu{0.4 m}$ $\ce{nh4cl}$, $\pu{0.1 mol}$ of $\ce{hcl}$. The. Hcl Added To Buffer.
From wisc.pb.unizin.org
Day 36 Buffer Solutions Chemistry 109, Fall 2020 Hcl Added To Buffer The standard practice is to assume that $\ce{hcl}$ (being a strong acid) reacts fully with the conjugate base in your buffer solution. The added \(hcl\) (a strong acid) or \(naoh\) (a strong base) will react completely with formate (a weak base) or formic acid (a. An ice chart is useful in determining the ph of the system after a strong. Hcl Added To Buffer.
From www.numerade.com
SOLVED Calculate the pH of the buffer solutions (show your Hcl Added To Buffer In more rigorous terms, buffer capacity is defined as the number of moles of an acid or base that has to be added to 1 liter of a buffer to cause its ph to change by 1 unit. An ice chart is useful in determining the ph of the system after a strong acid has been added. Adding the hcl. Hcl Added To Buffer.
From biochemmadeeasy.blogspot.com
Biochemistry Made Easy Weak Acids and Buffers Hcl Added To Buffer In a certain $\pu{1 l}$ buffer with $\pu{0.25 m}$ $\ce{nh3}$ and $\pu{0.4 m}$ $\ce{nh4cl}$, $\pu{0.1 mol}$ of $\ce{hcl}$. We can use a multiprotic weak acid to prepare buffers at as many different ph’s as there. Calculate the ph of a buffer before and after the addition of added acid or. 50.0 ml of 0.100 m hcl was added to. In. Hcl Added To Buffer.
From jackwestin.com
Buffers 2 Acid Base Equilibria MCAT Content Hcl Added To Buffer Adding the hcl (an acid) has changed the ph of the buffer in the acidic direction, from 9.294 to 9.014. 1) i will use 0.500 m hcl for (b). An ice chart is useful in determining the ph of the system after a strong acid has been added. In a certain $\pu{1 l}$ buffer with $\pu{0.25 m}$ $\ce{nh3}$ and $\pu{0.4. Hcl Added To Buffer.
From general.chemistrysteps.com
Titration of a Weak Base by a Strong Acid Chemistry Steps Hcl Added To Buffer We can use a multiprotic weak acid to prepare buffers at as many different ph’s as there. In a certain $\pu{1 l}$ buffer with $\pu{0.25 m}$ $\ce{nh3}$ and $\pu{0.4 m}$ $\ce{nh4cl}$, $\pu{0.1 mol}$ of $\ce{hcl}$. An ice chart is useful in determining the ph of the system after a strong acid has been added. In more rigorous terms, buffer capacity. Hcl Added To Buffer.
From www.youtube.com
Acids, Bases, pH, Buffers YouTube Hcl Added To Buffer Calculate the ph of a buffer before and after the addition of added acid or. 50.0 ml of 0.100 m hcl was added to. The standard practice is to assume that $\ce{hcl}$ (being a strong acid) reacts fully with the conjugate base in your buffer solution. As we expect, adding hcl decreases the buffer’s ph by a small amount, dropping. Hcl Added To Buffer.
From chem.libretexts.org
14.10 Buffers are Solutions that Resist pH Change Chemistry LibreTexts Hcl Added To Buffer In more rigorous terms, buffer capacity is defined as the number of moles of an acid or base that has to be added to 1 liter of a buffer to cause its ph to change by 1 unit. We can use a multiprotic weak acid to prepare buffers at as many different ph’s as there. 50.0 ml of 0.100 m. Hcl Added To Buffer.
From www.pinterest.com
Understanding Buffer Solution Chemistry A Complete Guide Hcl Added To Buffer Adding the hcl (an acid) has changed the ph of the buffer in the acidic direction, from 9.294 to 9.014. In more rigorous terms, buffer capacity is defined as the number of moles of an acid or base that has to be added to 1 liter of a buffer to cause its ph to change by 1 unit. 50.0 ml. Hcl Added To Buffer.
From criticalthinking.cloud
how to solve buffer problems chemistry Hcl Added To Buffer Adding the hcl (an acid) has changed the ph of the buffer in the acidic direction, from 9.294 to 9.014. 50.0 ml of 0.100 m hcl was added to. Calculate the ph of a buffer before and after the addition of added acid or. In a certain $\pu{1 l}$ buffer with $\pu{0.25 m}$ $\ce{nh3}$ and $\pu{0.4 m}$ $\ce{nh4cl}$, $\pu{0.1 mol}$. Hcl Added To Buffer.
From education-portal.com
AcidBase Buffers Calculating the pH of a Buffered Solution Video Hcl Added To Buffer We can use a multiprotic weak acid to prepare buffers at as many different ph’s as there. Calculate the ph of a buffer before and after the addition of added acid or. In more rigorous terms, buffer capacity is defined as the number of moles of an acid or base that has to be added to 1 liter of a. Hcl Added To Buffer.
From www.slideserve.com
PPT BUFFERS PowerPoint Presentation, free download ID2881472 Hcl Added To Buffer 50.0 ml of 0.100 m hcl was added to. 1) i will use 0.500 m hcl for (b). In a certain $\pu{1 l}$ buffer with $\pu{0.25 m}$ $\ce{nh3}$ and $\pu{0.4 m}$ $\ce{nh4cl}$, $\pu{0.1 mol}$ of $\ce{hcl}$. We can use a multiprotic weak acid to prepare buffers at as many different ph’s as there. Adding the hcl (an acid) has changed. Hcl Added To Buffer.
From www.youtube.com
Buffer solution pH calculations Chemistry Khan Academy YouTube Hcl Added To Buffer An ice chart is useful in determining the ph of the system after a strong acid has been added. The standard practice is to assume that $\ce{hcl}$ (being a strong acid) reacts fully with the conjugate base in your buffer solution. 50.0 ml of 0.100 m hcl was added to. Calculate the ph of a buffer before and after the. Hcl Added To Buffer.
From www.slideserve.com
PPT What is a buffer? PowerPoint Presentation, free download ID1149749 Hcl Added To Buffer Adding the hcl (an acid) has changed the ph of the buffer in the acidic direction, from 9.294 to 9.014. In a certain $\pu{1 l}$ buffer with $\pu{0.25 m}$ $\ce{nh3}$ and $\pu{0.4 m}$ $\ce{nh4cl}$, $\pu{0.1 mol}$ of $\ce{hcl}$. In more rigorous terms, buffer capacity is defined as the number of moles of an acid or base that has to be. Hcl Added To Buffer.