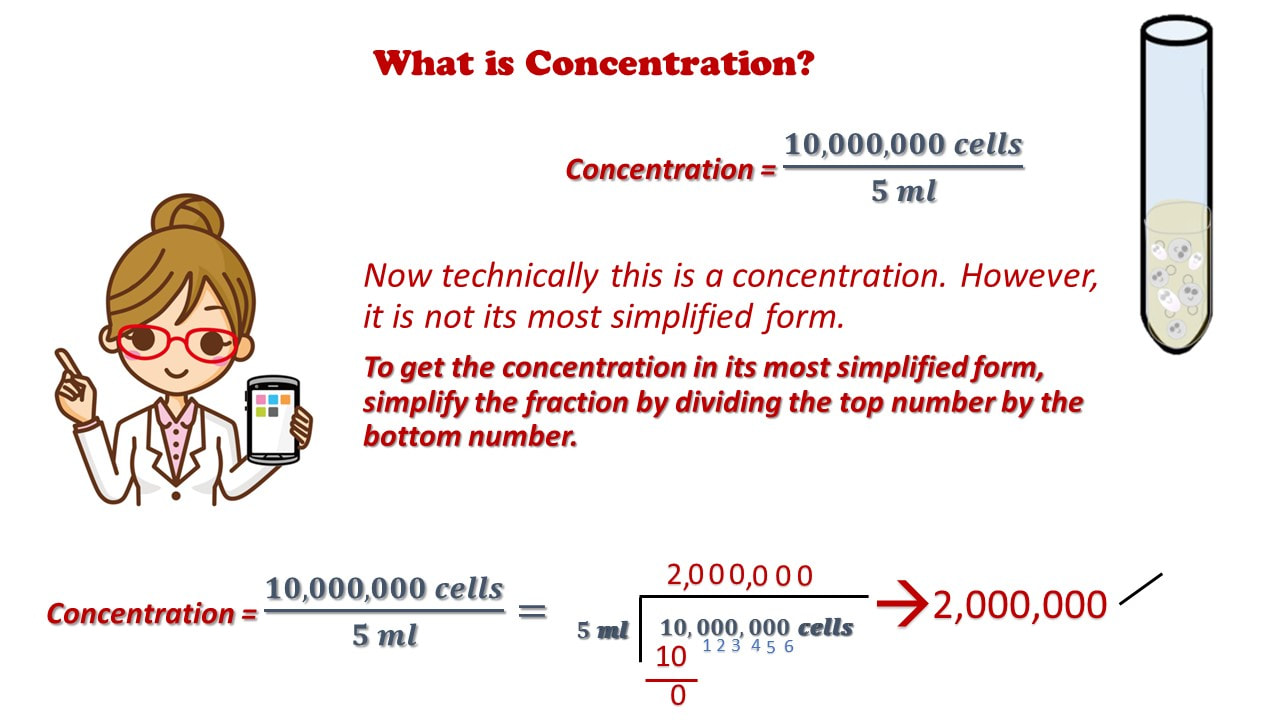
Dilution Chemistry Formula . An example of a dilute. Dilution is the process of adding a solvent to a solution to reduce the concentration of the solute. A dilution is a solution made by adding more solvent to a more concentrated solution (stock solution), which reduces the concentration of the solute. Concentration is the process of removing. The formula for calculating a dilution is (c1) (v1) = (c2) (v2) where. V1 is the volume of the starting solution. C1 is the concentration of the starting solution. We will begin our discussion of solution concentration with two related and relative terms: A dilute solution is one. State whether the concentration of a solution is directly or indirectly proportional to its volume.
from www.scientistcindy.com
V1 is the volume of the starting solution. The formula for calculating a dilution is (c1) (v1) = (c2) (v2) where. Dilution is the process of adding a solvent to a solution to reduce the concentration of the solute. State whether the concentration of a solution is directly or indirectly proportional to its volume. A dilute solution is one. An example of a dilute. Concentration is the process of removing. A dilution is a solution made by adding more solvent to a more concentrated solution (stock solution), which reduces the concentration of the solute. C1 is the concentration of the starting solution. We will begin our discussion of solution concentration with two related and relative terms:
Dilution Series and Calculations SCIENTIST CINDY
Dilution Chemistry Formula V1 is the volume of the starting solution. The formula for calculating a dilution is (c1) (v1) = (c2) (v2) where. Dilution is the process of adding a solvent to a solution to reduce the concentration of the solute. We will begin our discussion of solution concentration with two related and relative terms: A dilute solution is one. C1 is the concentration of the starting solution. V1 is the volume of the starting solution. Concentration is the process of removing. An example of a dilute. State whether the concentration of a solution is directly or indirectly proportional to its volume. A dilution is a solution made by adding more solvent to a more concentrated solution (stock solution), which reduces the concentration of the solute.
From www.showme.com
Dilution calculation Science, Chemistry ShowMe Dilution Chemistry Formula We will begin our discussion of solution concentration with two related and relative terms: State whether the concentration of a solution is directly or indirectly proportional to its volume. Concentration is the process of removing. A dilution is a solution made by adding more solvent to a more concentrated solution (stock solution), which reduces the concentration of the solute. An. Dilution Chemistry Formula.
From www.youtube.com
Dilution Chart.Helpful video. Understand how to prepare dilutions in Dilution Chemistry Formula C1 is the concentration of the starting solution. V1 is the volume of the starting solution. A dilution is a solution made by adding more solvent to a more concentrated solution (stock solution), which reduces the concentration of the solute. Concentration is the process of removing. A dilute solution is one. State whether the concentration of a solution is directly. Dilution Chemistry Formula.
From ar.inspiredpencil.com
Dilution Equation Dilution Chemistry Formula A dilution is a solution made by adding more solvent to a more concentrated solution (stock solution), which reduces the concentration of the solute. C1 is the concentration of the starting solution. An example of a dilute. State whether the concentration of a solution is directly or indirectly proportional to its volume. V1 is the volume of the starting solution.. Dilution Chemistry Formula.
From www.youtube.com
CHEMISTRY 101 Solution Dilutions YouTube Dilution Chemistry Formula Dilution is the process of adding a solvent to a solution to reduce the concentration of the solute. Concentration is the process of removing. C1 is the concentration of the starting solution. We will begin our discussion of solution concentration with two related and relative terms: A dilute solution is one. A dilution is a solution made by adding more. Dilution Chemistry Formula.
From www.youtube.com
Chem 11 Dilution Calculations_Solving for Final Volume after Dilution Dilution Chemistry Formula We will begin our discussion of solution concentration with two related and relative terms: An example of a dilute. A dilute solution is one. A dilution is a solution made by adding more solvent to a more concentrated solution (stock solution), which reduces the concentration of the solute. State whether the concentration of a solution is directly or indirectly proportional. Dilution Chemistry Formula.
From www.youtube.com
A Level Chemistry Dilution Calculations Worked Example YouTube Dilution Chemistry Formula V1 is the volume of the starting solution. We will begin our discussion of solution concentration with two related and relative terms: State whether the concentration of a solution is directly or indirectly proportional to its volume. An example of a dilute. Dilution is the process of adding a solvent to a solution to reduce the concentration of the solute.. Dilution Chemistry Formula.
From www.slideserve.com
PPT Preparing Solutions with Dilutions PowerPoint Presentation, free Dilution Chemistry Formula A dilution is a solution made by adding more solvent to a more concentrated solution (stock solution), which reduces the concentration of the solute. A dilute solution is one. V1 is the volume of the starting solution. Concentration is the process of removing. Dilution is the process of adding a solvent to a solution to reduce the concentration of the. Dilution Chemistry Formula.
From www.youtube.com
Molarity and Dilution YouTube Dilution Chemistry Formula We will begin our discussion of solution concentration with two related and relative terms: A dilute solution is one. Concentration is the process of removing. V1 is the volume of the starting solution. Dilution is the process of adding a solvent to a solution to reduce the concentration of the solute. An example of a dilute. C1 is the concentration. Dilution Chemistry Formula.
From www.scientistcindy.com
Dilution Series and Calculations SCIENTIST CINDY Dilution Chemistry Formula An example of a dilute. V1 is the volume of the starting solution. Concentration is the process of removing. A dilution is a solution made by adding more solvent to a more concentrated solution (stock solution), which reduces the concentration of the solute. A dilute solution is one. The formula for calculating a dilution is (c1) (v1) = (c2) (v2). Dilution Chemistry Formula.
From www.youtube.com
Dilution problems Chemistry Molarity & Concentration Examples Dilution Chemistry Formula Dilution is the process of adding a solvent to a solution to reduce the concentration of the solute. A dilution is a solution made by adding more solvent to a more concentrated solution (stock solution), which reduces the concentration of the solute. The formula for calculating a dilution is (c1) (v1) = (c2) (v2) where. A dilute solution is one.. Dilution Chemistry Formula.
From www.youtube.com
Quickvideo Using the dilution equation M1V1=M2V2 YouTube Dilution Chemistry Formula A dilute solution is one. V1 is the volume of the starting solution. Concentration is the process of removing. A dilution is a solution made by adding more solvent to a more concentrated solution (stock solution), which reduces the concentration of the solute. The formula for calculating a dilution is (c1) (v1) = (c2) (v2) where. Dilution is the process. Dilution Chemistry Formula.
From www.youtube.com
Chemistry 11 Dilution Calculations Solving for Initial Volume Example Dilution Chemistry Formula V1 is the volume of the starting solution. Concentration is the process of removing. We will begin our discussion of solution concentration with two related and relative terms: State whether the concentration of a solution is directly or indirectly proportional to its volume. Dilution is the process of adding a solvent to a solution to reduce the concentration of the. Dilution Chemistry Formula.
From www.youtube.com
How to Calculate Dilution Factor YouTube Dilution Chemistry Formula An example of a dilute. Concentration is the process of removing. We will begin our discussion of solution concentration with two related and relative terms: V1 is the volume of the starting solution. Dilution is the process of adding a solvent to a solution to reduce the concentration of the solute. A dilute solution is one. C1 is the concentration. Dilution Chemistry Formula.
From www.youtube.com
Chem121 Dilution Equation 8 5 YouTube Dilution Chemistry Formula The formula for calculating a dilution is (c1) (v1) = (c2) (v2) where. V1 is the volume of the starting solution. We will begin our discussion of solution concentration with two related and relative terms: A dilute solution is one. State whether the concentration of a solution is directly or indirectly proportional to its volume. Dilution is the process of. Dilution Chemistry Formula.
From microbenotes.com
Serial Dilution Formula, Calculator, Method, Uses, Examples Dilution Chemistry Formula An example of a dilute. C1 is the concentration of the starting solution. We will begin our discussion of solution concentration with two related and relative terms: A dilute solution is one. V1 is the volume of the starting solution. State whether the concentration of a solution is directly or indirectly proportional to its volume. A dilution is a solution. Dilution Chemistry Formula.
From www.youtube.com
Chem143 Dilution Equation YouTube Dilution Chemistry Formula Concentration is the process of removing. C1 is the concentration of the starting solution. We will begin our discussion of solution concentration with two related and relative terms: V1 is the volume of the starting solution. An example of a dilute. A dilute solution is one. State whether the concentration of a solution is directly or indirectly proportional to its. Dilution Chemistry Formula.
From www.medicine.mcgill.ca
Serial Dilutions Dilution Chemistry Formula A dilution is a solution made by adding more solvent to a more concentrated solution (stock solution), which reduces the concentration of the solute. Dilution is the process of adding a solvent to a solution to reduce the concentration of the solute. We will begin our discussion of solution concentration with two related and relative terms: C1 is the concentration. Dilution Chemistry Formula.
From www.youtube.com
Calculating Dilutions C1V1=C2V2 PowerPoint YouTube Dilution Chemistry Formula We will begin our discussion of solution concentration with two related and relative terms: Dilution is the process of adding a solvent to a solution to reduce the concentration of the solute. An example of a dilute. C1 is the concentration of the starting solution. The formula for calculating a dilution is (c1) (v1) = (c2) (v2) where. State whether. Dilution Chemistry Formula.
From chem.libretexts.org
5.2 Solutions and Dilutions Chemistry LibreTexts Dilution Chemistry Formula We will begin our discussion of solution concentration with two related and relative terms: Dilution is the process of adding a solvent to a solution to reduce the concentration of the solute. Concentration is the process of removing. State whether the concentration of a solution is directly or indirectly proportional to its volume. V1 is the volume of the starting. Dilution Chemistry Formula.
From www.youtube.com
Dilution Problems, Chemistry, Molarity & Concentration Examples Dilution Chemistry Formula State whether the concentration of a solution is directly or indirectly proportional to its volume. C1 is the concentration of the starting solution. We will begin our discussion of solution concentration with two related and relative terms: Concentration is the process of removing. A dilute solution is one. An example of a dilute. Dilution is the process of adding a. Dilution Chemistry Formula.
From www.slideshare.net
Molarity and dilution Dilution Chemistry Formula The formula for calculating a dilution is (c1) (v1) = (c2) (v2) where. C1 is the concentration of the starting solution. A dilute solution is one. Dilution is the process of adding a solvent to a solution to reduce the concentration of the solute. V1 is the volume of the starting solution. An example of a dilute. A dilution is. Dilution Chemistry Formula.
From www.youtube.com
Dilution formula and how to use it to solve problems. 3 examples solved Dilution Chemistry Formula We will begin our discussion of solution concentration with two related and relative terms: V1 is the volume of the starting solution. Dilution is the process of adding a solvent to a solution to reduce the concentration of the solute. State whether the concentration of a solution is directly or indirectly proportional to its volume. Concentration is the process of. Dilution Chemistry Formula.
From kayliefernandez.blogspot.com
PreAP Chemistry Dilutions Dilution Chemistry Formula V1 is the volume of the starting solution. C1 is the concentration of the starting solution. Concentration is the process of removing. A dilute solution is one. A dilution is a solution made by adding more solvent to a more concentrated solution (stock solution), which reduces the concentration of the solute. We will begin our discussion of solution concentration with. Dilution Chemistry Formula.
From fphoto.photoshelter.com
science chemistry solubility dilution Fundamental Photographs The Dilution Chemistry Formula State whether the concentration of a solution is directly or indirectly proportional to its volume. An example of a dilute. V1 is the volume of the starting solution. C1 is the concentration of the starting solution. We will begin our discussion of solution concentration with two related and relative terms: Concentration is the process of removing. A dilute solution is. Dilution Chemistry Formula.
From www.youtube.com
Dilution Problems Chemistry Tutorial YouTube Dilution Chemistry Formula Concentration is the process of removing. Dilution is the process of adding a solvent to a solution to reduce the concentration of the solute. The formula for calculating a dilution is (c1) (v1) = (c2) (v2) where. A dilute solution is one. State whether the concentration of a solution is directly or indirectly proportional to its volume. A dilution is. Dilution Chemistry Formula.
From chem2u.blogspot.com
chem2U Dilution method formula Dilution Chemistry Formula We will begin our discussion of solution concentration with two related and relative terms: C1 is the concentration of the starting solution. Dilution is the process of adding a solvent to a solution to reduce the concentration of the solute. The formula for calculating a dilution is (c1) (v1) = (c2) (v2) where. Concentration is the process of removing. An. Dilution Chemistry Formula.
From study.com
Dilution Definition, Equation & Factors Video & Lesson Transcript Dilution Chemistry Formula A dilution is a solution made by adding more solvent to a more concentrated solution (stock solution), which reduces the concentration of the solute. V1 is the volume of the starting solution. The formula for calculating a dilution is (c1) (v1) = (c2) (v2) where. Concentration is the process of removing. We will begin our discussion of solution concentration with. Dilution Chemistry Formula.
From www.expii.com
Dilution of Solutions — Overview & Examples Expii Dilution Chemistry Formula We will begin our discussion of solution concentration with two related and relative terms: A dilution is a solution made by adding more solvent to a more concentrated solution (stock solution), which reduces the concentration of the solute. C1 is the concentration of the starting solution. The formula for calculating a dilution is (c1) (v1) = (c2) (v2) where. V1. Dilution Chemistry Formula.
From www.youtube.com
Chemistry U9 Molarity and Dilution Calculations YouTube Dilution Chemistry Formula We will begin our discussion of solution concentration with two related and relative terms: A dilution is a solution made by adding more solvent to a more concentrated solution (stock solution), which reduces the concentration of the solute. An example of a dilute. The formula for calculating a dilution is (c1) (v1) = (c2) (v2) where. Dilution is the process. Dilution Chemistry Formula.
From www.youtube.com
Chemistry 11 Dilution Calculations Solving for Initial Concentration Dilution Chemistry Formula An example of a dilute. We will begin our discussion of solution concentration with two related and relative terms: A dilution is a solution made by adding more solvent to a more concentrated solution (stock solution), which reduces the concentration of the solute. Concentration is the process of removing. V1 is the volume of the starting solution. State whether the. Dilution Chemistry Formula.
From www.ck12.org
Dilution (M[i]V[i]=M[f]V[f]) Example 1 ( Video ) Chemistry CK12 Dilution Chemistry Formula We will begin our discussion of solution concentration with two related and relative terms: Concentration is the process of removing. A dilute solution is one. The formula for calculating a dilution is (c1) (v1) = (c2) (v2) where. State whether the concentration of a solution is directly or indirectly proportional to its volume. A dilution is a solution made by. Dilution Chemistry Formula.
From www.showme.com
Dilution calculation Science, Chemicalreactions, Biology ShowMe Dilution Chemistry Formula Dilution is the process of adding a solvent to a solution to reduce the concentration of the solute. State whether the concentration of a solution is directly or indirectly proportional to its volume. V1 is the volume of the starting solution. C1 is the concentration of the starting solution. We will begin our discussion of solution concentration with two related. Dilution Chemistry Formula.
From www.pdfprof.com
how to calculate dilution factor Dilution Chemistry Formula Dilution is the process of adding a solvent to a solution to reduce the concentration of the solute. State whether the concentration of a solution is directly or indirectly proportional to its volume. C1 is the concentration of the starting solution. An example of a dilute. Concentration is the process of removing. The formula for calculating a dilution is (c1). Dilution Chemistry Formula.
From www.slideserve.com
PPT Dilution Calculations PowerPoint Presentation, free download ID Dilution Chemistry Formula A dilute solution is one. State whether the concentration of a solution is directly or indirectly proportional to its volume. The formula for calculating a dilution is (c1) (v1) = (c2) (v2) where. An example of a dilute. Concentration is the process of removing. C1 is the concentration of the starting solution. Dilution is the process of adding a solvent. Dilution Chemistry Formula.
From www.youtube.com
TRU Chemistry Labs How To do Dilution Calculations YouTube Dilution Chemistry Formula V1 is the volume of the starting solution. The formula for calculating a dilution is (c1) (v1) = (c2) (v2) where. Concentration is the process of removing. A dilution is a solution made by adding more solvent to a more concentrated solution (stock solution), which reduces the concentration of the solute. An example of a dilute. C1 is the concentration. Dilution Chemistry Formula.