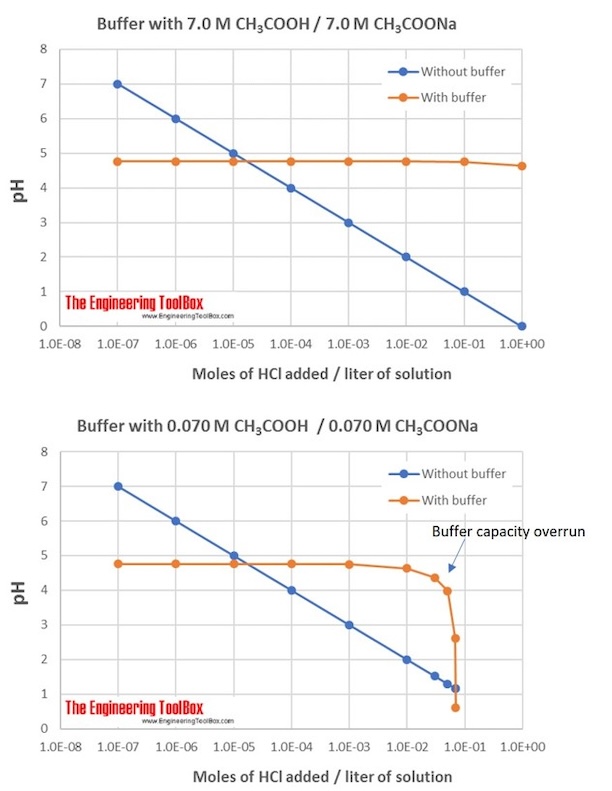
Buffer Capacity Example . If you remember high school chemistry or took a college course like chemistry 101, you will have conducted a titration test. the buffer capacity is a measure of resistant a particular solution is resistant to change in ph when an acid or a base is added to it. What factors determine the effectiveness (or capacity) of a buffer? The goal of a buffer is to keep the ph of a solution within a narrow range. Buffer capacity depends on the amounts of the weak acid and its conjugate base that are in a buffer mixture. the buffer capacity is the amount of acid or base that can be added to a given volume of a buffer solution before the ph changes significantly, usually by one. learn what buffer capacity is, and how to calculate it using a formula. See an example problem of a sodium phosphate buffer and practice problems with solutions. the definition of buffer capacity, and an example showing why it depends on the absolute concentrations of the conjugate. the buffer capacity is the amount of acid or base that can be added to a given volume of a buffer solution before the ph changes significantly, usually by one unit. the buffer capacity is the amount of acid or base that can be added to a given volume of a buffer solution before the ph changes.
from www.engineeringtoolbox.com
the buffer capacity is the amount of acid or base that can be added to a given volume of a buffer solution before the ph changes significantly, usually by one. The goal of a buffer is to keep the ph of a solution within a narrow range. learn what buffer capacity is, and how to calculate it using a formula. See an example problem of a sodium phosphate buffer and practice problems with solutions. the definition of buffer capacity, and an example showing why it depends on the absolute concentrations of the conjugate. the buffer capacity is a measure of resistant a particular solution is resistant to change in ph when an acid or a base is added to it. the buffer capacity is the amount of acid or base that can be added to a given volume of a buffer solution before the ph changes significantly, usually by one unit. the buffer capacity is the amount of acid or base that can be added to a given volume of a buffer solution before the ph changes. Buffer capacity depends on the amounts of the weak acid and its conjugate base that are in a buffer mixture. What factors determine the effectiveness (or capacity) of a buffer?
Buffer Solutions
Buffer Capacity Example the buffer capacity is the amount of acid or base that can be added to a given volume of a buffer solution before the ph changes significantly, usually by one unit. the buffer capacity is the amount of acid or base that can be added to a given volume of a buffer solution before the ph changes. the buffer capacity is the amount of acid or base that can be added to a given volume of a buffer solution before the ph changes significantly, usually by one. Buffer capacity depends on the amounts of the weak acid and its conjugate base that are in a buffer mixture. the buffer capacity is a measure of resistant a particular solution is resistant to change in ph when an acid or a base is added to it. the definition of buffer capacity, and an example showing why it depends on the absolute concentrations of the conjugate. If you remember high school chemistry or took a college course like chemistry 101, you will have conducted a titration test. See an example problem of a sodium phosphate buffer and practice problems with solutions. The goal of a buffer is to keep the ph of a solution within a narrow range. the buffer capacity is the amount of acid or base that can be added to a given volume of a buffer solution before the ph changes significantly, usually by one unit. What factors determine the effectiveness (or capacity) of a buffer? learn what buffer capacity is, and how to calculate it using a formula.
From www.youtube.com
Buffer Capacity YouTube Buffer Capacity Example the buffer capacity is the amount of acid or base that can be added to a given volume of a buffer solution before the ph changes. What factors determine the effectiveness (or capacity) of a buffer? The goal of a buffer is to keep the ph of a solution within a narrow range. the buffer capacity is the. Buffer Capacity Example.
From www.slideserve.com
PPT Chapter 13 Applications of Aqueous Equilibria PowerPoint Buffer Capacity Example learn what buffer capacity is, and how to calculate it using a formula. the definition of buffer capacity, and an example showing why it depends on the absolute concentrations of the conjugate. Buffer capacity depends on the amounts of the weak acid and its conjugate base that are in a buffer mixture. If you remember high school chemistry. Buffer Capacity Example.
From www.studypool.com
SOLUTION 4 buffering capacity Studypool Buffer Capacity Example If you remember high school chemistry or took a college course like chemistry 101, you will have conducted a titration test. What factors determine the effectiveness (or capacity) of a buffer? See an example problem of a sodium phosphate buffer and practice problems with solutions. the buffer capacity is the amount of acid or base that can be added. Buffer Capacity Example.
From www.slideserve.com
PPT Chemistry 100 Chapter 17 PowerPoint Presentation, free download Buffer Capacity Example Buffer capacity depends on the amounts of the weak acid and its conjugate base that are in a buffer mixture. the definition of buffer capacity, and an example showing why it depends on the absolute concentrations of the conjugate. The goal of a buffer is to keep the ph of a solution within a narrow range. What factors determine. Buffer Capacity Example.
From en.ppt-online.org
Solutions. Acidbase equilibrium in biological systems online Buffer Capacity Example the buffer capacity is the amount of acid or base that can be added to a given volume of a buffer solution before the ph changes significantly, usually by one. See an example problem of a sodium phosphate buffer and practice problems with solutions. What factors determine the effectiveness (or capacity) of a buffer? Buffer capacity depends on the. Buffer Capacity Example.
From www.slideserve.com
PPT Effect of Buffer Capacity PowerPoint Presentation, free download Buffer Capacity Example learn what buffer capacity is, and how to calculate it using a formula. the definition of buffer capacity, and an example showing why it depends on the absolute concentrations of the conjugate. The goal of a buffer is to keep the ph of a solution within a narrow range. the buffer capacity is a measure of resistant. Buffer Capacity Example.
From www.slideserve.com
PPT Buffers & Buffer Capacity PowerPoint Presentation ID5949383 Buffer Capacity Example The goal of a buffer is to keep the ph of a solution within a narrow range. What factors determine the effectiveness (or capacity) of a buffer? the buffer capacity is the amount of acid or base that can be added to a given volume of a buffer solution before the ph changes. If you remember high school chemistry. Buffer Capacity Example.
From www.slideserve.com
PPT Buffering Capacity Addition of STRONG Acids or Bases PowerPoint Buffer Capacity Example the definition of buffer capacity, and an example showing why it depends on the absolute concentrations of the conjugate. the buffer capacity is a measure of resistant a particular solution is resistant to change in ph when an acid or a base is added to it. the buffer capacity is the amount of acid or base that. Buffer Capacity Example.
From www.slideserve.com
PPT Determination of Buffer Capacity PowerPoint Presentation, free Buffer Capacity Example the definition of buffer capacity, and an example showing why it depends on the absolute concentrations of the conjugate. If you remember high school chemistry or took a college course like chemistry 101, you will have conducted a titration test. Buffer capacity depends on the amounts of the weak acid and its conjugate base that are in a buffer. Buffer Capacity Example.
From www.slideserve.com
PPT Determination of Buffer Capacity PowerPoint Presentation, free Buffer Capacity Example What factors determine the effectiveness (or capacity) of a buffer? learn what buffer capacity is, and how to calculate it using a formula. Buffer capacity depends on the amounts of the weak acid and its conjugate base that are in a buffer mixture. the buffer capacity is the amount of acid or base that can be added to. Buffer Capacity Example.
From www.slideserve.com
PPT Chapter 15 PowerPoint Presentation, free download ID844461 Buffer Capacity Example Buffer capacity depends on the amounts of the weak acid and its conjugate base that are in a buffer mixture. the buffer capacity is the amount of acid or base that can be added to a given volume of a buffer solution before the ph changes significantly, usually by one unit. What factors determine the effectiveness (or capacity) of. Buffer Capacity Example.
From www.slideserve.com
PPT Buffers & Buffer Capacity PowerPoint Presentation, free download Buffer Capacity Example learn what buffer capacity is, and how to calculate it using a formula. The goal of a buffer is to keep the ph of a solution within a narrow range. the buffer capacity is a measure of resistant a particular solution is resistant to change in ph when an acid or a base is added to it. Buffer. Buffer Capacity Example.
From www.scienceabc.com
Buffer Capacity Definition And How To Calculate It Buffer Capacity Example Buffer capacity depends on the amounts of the weak acid and its conjugate base that are in a buffer mixture. See an example problem of a sodium phosphate buffer and practice problems with solutions. the buffer capacity is the amount of acid or base that can be added to a given volume of a buffer solution before the ph. Buffer Capacity Example.
From www.slideserve.com
PPT pH and Buffers PowerPoint Presentation, free download ID6910668 Buffer Capacity Example If you remember high school chemistry or took a college course like chemistry 101, you will have conducted a titration test. learn what buffer capacity is, and how to calculate it using a formula. The goal of a buffer is to keep the ph of a solution within a narrow range. the buffer capacity is the amount of. Buffer Capacity Example.
From studylib.net
Buffer Capacity Buffer Capacity Example the buffer capacity is the amount of acid or base that can be added to a given volume of a buffer solution before the ph changes. the definition of buffer capacity, and an example showing why it depends on the absolute concentrations of the conjugate. the buffer capacity is a measure of resistant a particular solution is. Buffer Capacity Example.
From www.slideserve.com
PPT Chemical Equilibria PowerPoint Presentation, free download ID Buffer Capacity Example the buffer capacity is the amount of acid or base that can be added to a given volume of a buffer solution before the ph changes significantly, usually by one. If you remember high school chemistry or took a college course like chemistry 101, you will have conducted a titration test. See an example problem of a sodium phosphate. Buffer Capacity Example.
From serc.carleton.edu
Buffer Capacity in Chemical Equilibrium How long can you Buffer Capacity Example the buffer capacity is a measure of resistant a particular solution is resistant to change in ph when an acid or a base is added to it. the buffer capacity is the amount of acid or base that can be added to a given volume of a buffer solution before the ph changes significantly, usually by one. . Buffer Capacity Example.
From www.slideserve.com
PPT Chapter 17 Additional Aqueous Equilibria PowerPoint Presentation Buffer Capacity Example the definition of buffer capacity, and an example showing why it depends on the absolute concentrations of the conjugate. What factors determine the effectiveness (or capacity) of a buffer? the buffer capacity is a measure of resistant a particular solution is resistant to change in ph when an acid or a base is added to it. See an. Buffer Capacity Example.
From www.slideserve.com
PPT Determination of Buffer Capacity PowerPoint Presentation, free Buffer Capacity Example Buffer capacity depends on the amounts of the weak acid and its conjugate base that are in a buffer mixture. What factors determine the effectiveness (or capacity) of a buffer? the definition of buffer capacity, and an example showing why it depends on the absolute concentrations of the conjugate. the buffer capacity is the amount of acid or. Buffer Capacity Example.
From www.youtube.com
Buffer Capacity Concept & Calculation of Buffer Capacity Grade 11 Buffer Capacity Example the buffer capacity is the amount of acid or base that can be added to a given volume of a buffer solution before the ph changes. The goal of a buffer is to keep the ph of a solution within a narrow range. Buffer capacity depends on the amounts of the weak acid and its conjugate base that are. Buffer Capacity Example.
From www.slideserve.com
PPT Buffer PowerPoint Presentation, free download ID6831184 Buffer Capacity Example the buffer capacity is the amount of acid or base that can be added to a given volume of a buffer solution before the ph changes significantly, usually by one unit. See an example problem of a sodium phosphate buffer and practice problems with solutions. learn what buffer capacity is, and how to calculate it using a formula.. Buffer Capacity Example.
From chemexpert.net
Mastering Buffer Capacity Your Guide To Maintaining Chemical Harmony Buffer Capacity Example the buffer capacity is the amount of acid or base that can be added to a given volume of a buffer solution before the ph changes. learn what buffer capacity is, and how to calculate it using a formula. the buffer capacity is the amount of acid or base that can be added to a given volume. Buffer Capacity Example.
From www.slideserve.com
PPT Buffer Capacity Lab PowerPoint Presentation, free download ID Buffer Capacity Example the buffer capacity is the amount of acid or base that can be added to a given volume of a buffer solution before the ph changes. If you remember high school chemistry or took a college course like chemistry 101, you will have conducted a titration test. See an example problem of a sodium phosphate buffer and practice problems. Buffer Capacity Example.
From study.com
Identifying Buffer Capacity Chemistry Buffer Capacity Example the definition of buffer capacity, and an example showing why it depends on the absolute concentrations of the conjugate. the buffer capacity is the amount of acid or base that can be added to a given volume of a buffer solution before the ph changes significantly, usually by one. Buffer capacity depends on the amounts of the weak. Buffer Capacity Example.
From www.youtube.com
Buffer capacity Acids and bases AP Chemistry Khan Academy YouTube Buffer Capacity Example the buffer capacity is the amount of acid or base that can be added to a given volume of a buffer solution before the ph changes significantly, usually by one unit. the buffer capacity is a measure of resistant a particular solution is resistant to change in ph when an acid or a base is added to it.. Buffer Capacity Example.
From www.youtube.com
Buffer Capacity 03 Buffers in pharmaceutical and biological systems Buffer Capacity Example What factors determine the effectiveness (or capacity) of a buffer? The goal of a buffer is to keep the ph of a solution within a narrow range. the buffer capacity is a measure of resistant a particular solution is resistant to change in ph when an acid or a base is added to it. Buffer capacity depends on the. Buffer Capacity Example.
From www.engineeringtoolbox.com
Buffer Solutions Buffer Capacity Example the buffer capacity is the amount of acid or base that can be added to a given volume of a buffer solution before the ph changes significantly, usually by one. learn what buffer capacity is, and how to calculate it using a formula. The goal of a buffer is to keep the ph of a solution within a. Buffer Capacity Example.
From thenoveldifference.com
Buffer Action and Buffer Capacity The best 4 difference Buffer Capacity Example If you remember high school chemistry or took a college course like chemistry 101, you will have conducted a titration test. learn what buffer capacity is, and how to calculate it using a formula. the buffer capacity is the amount of acid or base that can be added to a given volume of a buffer solution before the. Buffer Capacity Example.
From exojbdfvv.blob.core.windows.net
What Is Biological Buffer System at Maureen Masters blog Buffer Capacity Example the buffer capacity is the amount of acid or base that can be added to a given volume of a buffer solution before the ph changes significantly, usually by one. the buffer capacity is a measure of resistant a particular solution is resistant to change in ph when an acid or a base is added to it. The. Buffer Capacity Example.
From www.slideserve.com
PPT Chapter 15 PowerPoint Presentation, free download ID4502040 Buffer Capacity Example the buffer capacity is the amount of acid or base that can be added to a given volume of a buffer solution before the ph changes significantly, usually by one unit. the buffer capacity is the amount of acid or base that can be added to a given volume of a buffer solution before the ph changes. What. Buffer Capacity Example.
From www.youtube.com
Buffer capacity; making a buffer with a given pH value YouTube Buffer Capacity Example the buffer capacity is a measure of resistant a particular solution is resistant to change in ph when an acid or a base is added to it. If you remember high school chemistry or took a college course like chemistry 101, you will have conducted a titration test. the buffer capacity is the amount of acid or base. Buffer Capacity Example.
From www.slideserve.com
PPT Buffering Capacity Addition of STRONG Acids or Bases PowerPoint Buffer Capacity Example Buffer capacity depends on the amounts of the weak acid and its conjugate base that are in a buffer mixture. If you remember high school chemistry or took a college course like chemistry 101, you will have conducted a titration test. See an example problem of a sodium phosphate buffer and practice problems with solutions. The goal of a buffer. Buffer Capacity Example.
From pharmacyscope.com
How do you calculate buffer capacity? Pharmacy Scope Buffer Capacity Example The goal of a buffer is to keep the ph of a solution within a narrow range. the definition of buffer capacity, and an example showing why it depends on the absolute concentrations of the conjugate. the buffer capacity is a measure of resistant a particular solution is resistant to change in ph when an acid or a. Buffer Capacity Example.
From www.slideserve.com
PPT Buffers PowerPoint Presentation, free download ID5124400 Buffer Capacity Example the buffer capacity is a measure of resistant a particular solution is resistant to change in ph when an acid or a base is added to it. the buffer capacity is the amount of acid or base that can be added to a given volume of a buffer solution before the ph changes significantly, usually by one. See. Buffer Capacity Example.
From www.researchgate.net
6. Schematic diagram of the buffer capacity of an aqueous system Buffer Capacity Example learn what buffer capacity is, and how to calculate it using a formula. the buffer capacity is the amount of acid or base that can be added to a given volume of a buffer solution before the ph changes. Buffer capacity depends on the amounts of the weak acid and its conjugate base that are in a buffer. Buffer Capacity Example.