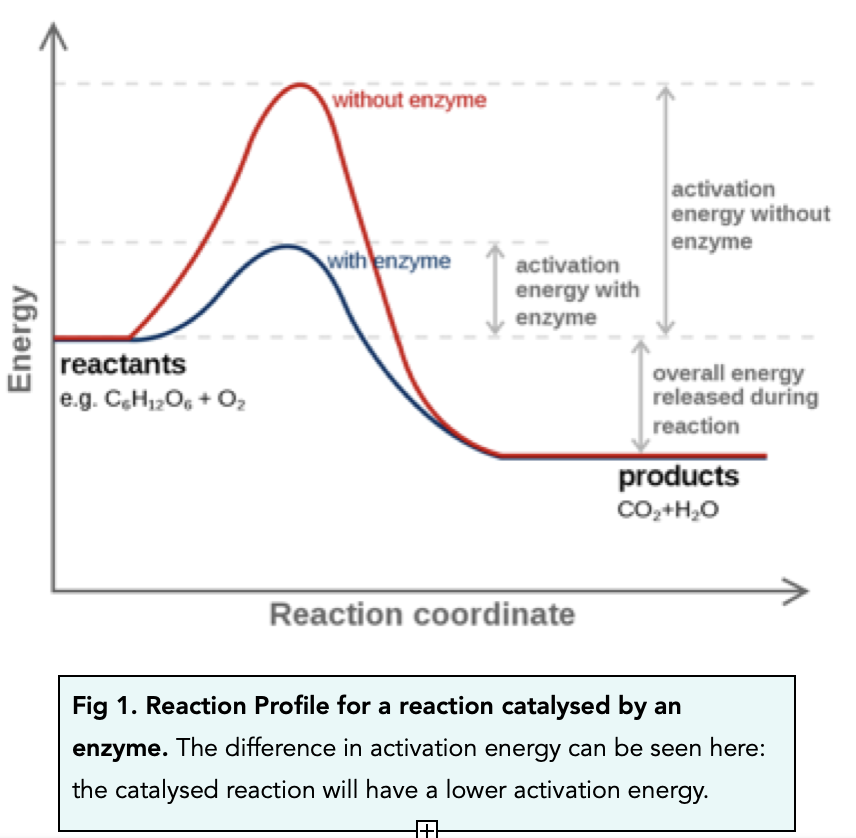
Catalyst Chemistry Term . Catalysts can be homogenous (in the same phase as the. what are catalysts, and how do they work in terms altering the parameters of a reaction? Describe the similarities and differences. catalyst, in chemistry, any substance that increases the rate of a reaction without itself being consumed. In other words, a catalyst is both a. With a helping hand from a catalyst, molecules that might take years to interact can now do. catalysts affect the rate of a chemical reaction by altering its mechanism to provide a lower activation energy. catalysts participate in a chemical reaction and increase its rate. They do not appear in the reaction’s net equation and are not consumed during the. a catalyst, in turn, is a substance that is not consumed by the chemical reaction, but acts to lower its activation energy. a catalyst is some material that speeds up chemical reactions.
from studymind.co.uk
Catalysts can be homogenous (in the same phase as the. a catalyst is some material that speeds up chemical reactions. what are catalysts, and how do they work in terms altering the parameters of a reaction? a catalyst, in turn, is a substance that is not consumed by the chemical reaction, but acts to lower its activation energy. They do not appear in the reaction’s net equation and are not consumed during the. catalyst, in chemistry, any substance that increases the rate of a reaction without itself being consumed. Describe the similarities and differences. In other words, a catalyst is both a. catalysts affect the rate of a chemical reaction by altering its mechanism to provide a lower activation energy. catalysts participate in a chemical reaction and increase its rate.
Catalysts (GCSE Chemistry) Study Mind
Catalyst Chemistry Term They do not appear in the reaction’s net equation and are not consumed during the. what are catalysts, and how do they work in terms altering the parameters of a reaction? catalysts affect the rate of a chemical reaction by altering its mechanism to provide a lower activation energy. catalysts participate in a chemical reaction and increase its rate. a catalyst is some material that speeds up chemical reactions. They do not appear in the reaction’s net equation and are not consumed during the. Catalysts can be homogenous (in the same phase as the. In other words, a catalyst is both a. Describe the similarities and differences. catalyst, in chemistry, any substance that increases the rate of a reaction without itself being consumed. With a helping hand from a catalyst, molecules that might take years to interact can now do. a catalyst, in turn, is a substance that is not consumed by the chemical reaction, but acts to lower its activation energy.
From www.youtube.com
Identifying catalysts in a reaction YouTube Catalyst Chemistry Term catalysts participate in a chemical reaction and increase its rate. a catalyst is some material that speeds up chemical reactions. a catalyst, in turn, is a substance that is not consumed by the chemical reaction, but acts to lower its activation energy. Describe the similarities and differences. They do not appear in the reaction’s net equation and. Catalyst Chemistry Term.
From www.nagwa.com
Lesson Catalysts Nagwa Catalyst Chemistry Term catalyst, in chemistry, any substance that increases the rate of a reaction without itself being consumed. catalysts affect the rate of a chemical reaction by altering its mechanism to provide a lower activation energy. a catalyst, in turn, is a substance that is not consumed by the chemical reaction, but acts to lower its activation energy. Catalysts. Catalyst Chemistry Term.
From www.youtube.com
R2.2.6 Intermediates and catalysts (HL) YouTube Catalyst Chemistry Term Catalysts can be homogenous (in the same phase as the. what are catalysts, and how do they work in terms altering the parameters of a reaction? In other words, a catalyst is both a. a catalyst, in turn, is a substance that is not consumed by the chemical reaction, but acts to lower its activation energy. catalyst,. Catalyst Chemistry Term.
From examples.yourdictionary.com
Examples of Catalysts YourDictionary Catalyst Chemistry Term They do not appear in the reaction’s net equation and are not consumed during the. catalyst, in chemistry, any substance that increases the rate of a reaction without itself being consumed. Describe the similarities and differences. In other words, a catalyst is both a. a catalyst is some material that speeds up chemical reactions. a catalyst, in. Catalyst Chemistry Term.
From www.slideserve.com
PPT Catalyst PowerPoint Presentation ID4503154 Catalyst Chemistry Term a catalyst is some material that speeds up chemical reactions. In other words, a catalyst is both a. They do not appear in the reaction’s net equation and are not consumed during the. catalyst, in chemistry, any substance that increases the rate of a reaction without itself being consumed. a catalyst, in turn, is a substance that. Catalyst Chemistry Term.
From www.slideserve.com
PPT Chapter 11 Chemical Reactions PowerPoint Presentation, free Catalyst Chemistry Term They do not appear in the reaction’s net equation and are not consumed during the. a catalyst is some material that speeds up chemical reactions. Describe the similarities and differences. a catalyst, in turn, is a substance that is not consumed by the chemical reaction, but acts to lower its activation energy. catalyst, in chemistry, any substance. Catalyst Chemistry Term.
From exoddixpp.blob.core.windows.net
Define Catalyst Science Term at Michael Moorehead blog Catalyst Chemistry Term a catalyst, in turn, is a substance that is not consumed by the chemical reaction, but acts to lower its activation energy. In other words, a catalyst is both a. Catalysts can be homogenous (in the same phase as the. catalyst, in chemistry, any substance that increases the rate of a reaction without itself being consumed. With a. Catalyst Chemistry Term.
From www.slideshare.net
Catalyst Catalyst Chemistry Term They do not appear in the reaction’s net equation and are not consumed during the. a catalyst is some material that speeds up chemical reactions. catalyst, in chemistry, any substance that increases the rate of a reaction without itself being consumed. catalysts affect the rate of a chemical reaction by altering its mechanism to provide a lower. Catalyst Chemistry Term.
From www.slideserve.com
PPT Catalyst PowerPoint Presentation, free download ID3996834 Catalyst Chemistry Term what are catalysts, and how do they work in terms altering the parameters of a reaction? In other words, a catalyst is both a. They do not appear in the reaction’s net equation and are not consumed during the. catalysts affect the rate of a chemical reaction by altering its mechanism to provide a lower activation energy. . Catalyst Chemistry Term.
From www.slideserve.com
PPT Starter 1)Definition of catalysts 2) Difference between Catalyst Chemistry Term a catalyst, in turn, is a substance that is not consumed by the chemical reaction, but acts to lower its activation energy. Catalysts can be homogenous (in the same phase as the. a catalyst is some material that speeds up chemical reactions. catalysts affect the rate of a chemical reaction by altering its mechanism to provide a. Catalyst Chemistry Term.
From sciencenotes.org
What Is a Catalyst? Understand Catalysis Catalyst Chemistry Term Describe the similarities and differences. Catalysts can be homogenous (in the same phase as the. catalyst, in chemistry, any substance that increases the rate of a reaction without itself being consumed. With a helping hand from a catalyst, molecules that might take years to interact can now do. They do not appear in the reaction’s net equation and are. Catalyst Chemistry Term.
From www.researchgate.net
Reaction coordinate diagram showing the working principle of a catalyst Catalyst Chemistry Term a catalyst, in turn, is a substance that is not consumed by the chemical reaction, but acts to lower its activation energy. a catalyst is some material that speeds up chemical reactions. catalyst, in chemistry, any substance that increases the rate of a reaction without itself being consumed. With a helping hand from a catalyst, molecules that. Catalyst Chemistry Term.
From scitechdaily.com
Science Made Simple What Are Catalysts? Catalyst Chemistry Term With a helping hand from a catalyst, molecules that might take years to interact can now do. catalysts affect the rate of a chemical reaction by altering its mechanism to provide a lower activation energy. They do not appear in the reaction’s net equation and are not consumed during the. Catalysts can be homogenous (in the same phase as. Catalyst Chemistry Term.
From www.youtube.com
036Chemistry of Catalysis YouTube Catalyst Chemistry Term catalysts affect the rate of a chemical reaction by altering its mechanism to provide a lower activation energy. They do not appear in the reaction’s net equation and are not consumed during the. catalysts participate in a chemical reaction and increase its rate. a catalyst is some material that speeds up chemical reactions. a catalyst, in. Catalyst Chemistry Term.
From studymind.co.uk
Catalysts (GCSE Chemistry) Study Mind Catalyst Chemistry Term a catalyst, in turn, is a substance that is not consumed by the chemical reaction, but acts to lower its activation energy. They do not appear in the reaction’s net equation and are not consumed during the. catalyst, in chemistry, any substance that increases the rate of a reaction without itself being consumed. catalysts participate in a. Catalyst Chemistry Term.
From www.slideserve.com
PPT Mechanisms of Catalytic Reactions and Characterization of Catalyst Chemistry Term catalysts participate in a chemical reaction and increase its rate. In other words, a catalyst is both a. Describe the similarities and differences. a catalyst is some material that speeds up chemical reactions. a catalyst, in turn, is a substance that is not consumed by the chemical reaction, but acts to lower its activation energy. catalyst,. Catalyst Chemistry Term.
From www.slideserve.com
PPT Catalysts PowerPoint Presentation, free download ID2684800 Catalyst Chemistry Term a catalyst is some material that speeds up chemical reactions. catalyst, in chemistry, any substance that increases the rate of a reaction without itself being consumed. catalysts participate in a chemical reaction and increase its rate. They do not appear in the reaction’s net equation and are not consumed during the. In other words, a catalyst is. Catalyst Chemistry Term.
From www.chemengonline.com
Catalysis Fundamentals Chemical Engineering Page 1 Catalyst Chemistry Term Catalysts can be homogenous (in the same phase as the. catalysts affect the rate of a chemical reaction by altering its mechanism to provide a lower activation energy. Describe the similarities and differences. In other words, a catalyst is both a. With a helping hand from a catalyst, molecules that might take years to interact can now do. . Catalyst Chemistry Term.
From www.sciencelearn.org.nz
Chemical reactions and catalysts — Science Learning Hub Catalyst Chemistry Term what are catalysts, and how do they work in terms altering the parameters of a reaction? Catalysts can be homogenous (in the same phase as the. catalysts participate in a chemical reaction and increase its rate. catalysts affect the rate of a chemical reaction by altering its mechanism to provide a lower activation energy. catalyst, in. Catalyst Chemistry Term.
From www.researchgate.net
1 Schematic illustration of a catalytic process showing "A" and "B Catalyst Chemistry Term With a helping hand from a catalyst, molecules that might take years to interact can now do. catalyst, in chemistry, any substance that increases the rate of a reaction without itself being consumed. Catalysts can be homogenous (in the same phase as the. a catalyst, in turn, is a substance that is not consumed by the chemical reaction,. Catalyst Chemistry Term.
From courses.lumenlearning.com
Catalysis Chemistry Catalyst Chemistry Term catalyst, in chemistry, any substance that increases the rate of a reaction without itself being consumed. what are catalysts, and how do they work in terms altering the parameters of a reaction? catalysts affect the rate of a chemical reaction by altering its mechanism to provide a lower activation energy. Catalysts can be homogenous (in the same. Catalyst Chemistry Term.
From www.youtube.com
Catalysts AP Chemistry Khan Academy YouTube Catalyst Chemistry Term catalyst, in chemistry, any substance that increases the rate of a reaction without itself being consumed. a catalyst, in turn, is a substance that is not consumed by the chemical reaction, but acts to lower its activation energy. They do not appear in the reaction’s net equation and are not consumed during the. In other words, a catalyst. Catalyst Chemistry Term.
From gamesmartz.com
Catalyst Definition & Image GameSmartz Catalyst Chemistry Term Describe the similarities and differences. catalysts participate in a chemical reaction and increase its rate. They do not appear in the reaction’s net equation and are not consumed during the. With a helping hand from a catalyst, molecules that might take years to interact can now do. catalysts affect the rate of a chemical reaction by altering its. Catalyst Chemistry Term.
From www.youtube.com
What Are Catalysts? Reactions Chemistry FuseSchool YouTube Catalyst Chemistry Term catalysts participate in a chemical reaction and increase its rate. a catalyst, in turn, is a substance that is not consumed by the chemical reaction, but acts to lower its activation energy. Catalysts can be homogenous (in the same phase as the. a catalyst is some material that speeds up chemical reactions. In other words, a catalyst. Catalyst Chemistry Term.
From courses.lumenlearning.com
Catalysis Chemistry for Majors Catalyst Chemistry Term With a helping hand from a catalyst, molecules that might take years to interact can now do. a catalyst is some material that speeds up chemical reactions. catalyst, in chemistry, any substance that increases the rate of a reaction without itself being consumed. Catalysts can be homogenous (in the same phase as the. catalysts affect the rate. Catalyst Chemistry Term.
From dokumen.tips
(PPT) Catalyst. Homogeneous catalysis Homogeneous catalysis is a Catalyst Chemistry Term catalyst, in chemistry, any substance that increases the rate of a reaction without itself being consumed. In other words, a catalyst is both a. a catalyst, in turn, is a substance that is not consumed by the chemical reaction, but acts to lower its activation energy. what are catalysts, and how do they work in terms altering. Catalyst Chemistry Term.
From www.thoughtco.com
Catalysis Definition in Chemistry Catalyst Chemistry Term catalysts participate in a chemical reaction and increase its rate. Catalysts can be homogenous (in the same phase as the. In other words, a catalyst is both a. a catalyst, in turn, is a substance that is not consumed by the chemical reaction, but acts to lower its activation energy. a catalyst is some material that speeds. Catalyst Chemistry Term.
From www.britannica.com
Catalyst Examples, Definition, & Facts Britannica Catalyst Chemistry Term Catalysts can be homogenous (in the same phase as the. They do not appear in the reaction’s net equation and are not consumed during the. In other words, a catalyst is both a. With a helping hand from a catalyst, molecules that might take years to interact can now do. Describe the similarities and differences. what are catalysts, and. Catalyst Chemistry Term.
From www.slideserve.com
PPT Catalyst PowerPoint Presentation, free download ID3996834 Catalyst Chemistry Term a catalyst, in turn, is a substance that is not consumed by the chemical reaction, but acts to lower its activation energy. With a helping hand from a catalyst, molecules that might take years to interact can now do. They do not appear in the reaction’s net equation and are not consumed during the. what are catalysts, and. Catalyst Chemistry Term.
From medicaltaste.weebly.com
Periodic table catalyst definition chemistry medicaltaste Catalyst Chemistry Term a catalyst, in turn, is a substance that is not consumed by the chemical reaction, but acts to lower its activation energy. Catalysts can be homogenous (in the same phase as the. what are catalysts, and how do they work in terms altering the parameters of a reaction? catalysts affect the rate of a chemical reaction by. Catalyst Chemistry Term.
From www.chemistrystudent.com
Heterogeneous Catalysis (ALevel) ChemistryStudent Catalyst Chemistry Term catalyst, in chemistry, any substance that increases the rate of a reaction without itself being consumed. With a helping hand from a catalyst, molecules that might take years to interact can now do. Catalysts can be homogenous (in the same phase as the. Describe the similarities and differences. In other words, a catalyst is both a. a catalyst. Catalyst Chemistry Term.
From www.researchgate.net
Catalytic processes on a solid catalyst. Download Scientific Diagram Catalyst Chemistry Term a catalyst, in turn, is a substance that is not consumed by the chemical reaction, but acts to lower its activation energy. catalysts participate in a chemical reaction and increase its rate. catalysts affect the rate of a chemical reaction by altering its mechanism to provide a lower activation energy. catalyst, in chemistry, any substance that. Catalyst Chemistry Term.
From www.labunlimited.com
Solid Phase Catalysis in Continuous Flow Chemistry Lab Unlimited Catalyst Chemistry Term Describe the similarities and differences. Catalysts can be homogenous (in the same phase as the. a catalyst, in turn, is a substance that is not consumed by the chemical reaction, but acts to lower its activation energy. In other words, a catalyst is both a. what are catalysts, and how do they work in terms altering the parameters. Catalyst Chemistry Term.
From www.youtube.com
Homogeneous vs Heterogeneous Catalysts Basic Introduction YouTube Catalyst Chemistry Term They do not appear in the reaction’s net equation and are not consumed during the. catalysts participate in a chemical reaction and increase its rate. what are catalysts, and how do they work in terms altering the parameters of a reaction? In other words, a catalyst is both a. With a helping hand from a catalyst, molecules that. Catalyst Chemistry Term.
From thechemistrynotes.com
Catalyst and Catalysis Types, Examples, Differences Catalyst Chemistry Term catalysts participate in a chemical reaction and increase its rate. a catalyst, in turn, is a substance that is not consumed by the chemical reaction, but acts to lower its activation energy. catalysts affect the rate of a chemical reaction by altering its mechanism to provide a lower activation energy. They do not appear in the reaction’s. Catalyst Chemistry Term.