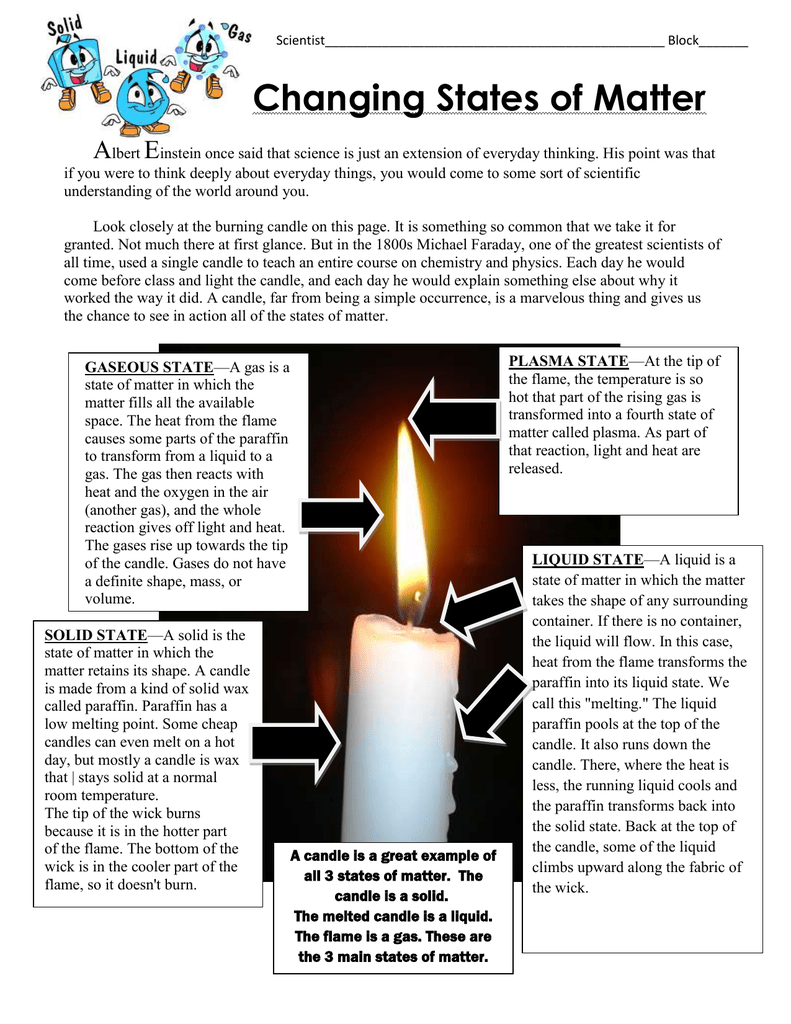
Is Lighting A Candle Chemical Change . color changes indicate chemical change. Physical and chemical changes with examples. before lighting the candle, make three qualitative observations and three quantitative observations of the candle. A physical change, such as a state change or dissolving, does not create a new substance, but a chemical change does. light is often the result of combustion or heating, or both. Melting wax vs burning candle. The wick absorbs the liquid wax, and it travels up the wick to the flame, where it combusts, keeping the flame going. Fe +o2 → fe2o3 fe + o 2 → fe 2 o 3. all the light a candle makes comes from a chemical reaction known as combustion in which the wax (made from. Salt in water vs burning magnesium. When you light a candle wick, you’ve started complex chemical reaction that will keep itself alive as long as the candle is intact and the flame is fed. First, the heat from the flame melts the wax nearby. lighting a candle.
from studylib.net
lighting a candle. The wick absorbs the liquid wax, and it travels up the wick to the flame, where it combusts, keeping the flame going. Physical and chemical changes with examples. Melting wax vs burning candle. When you light a candle wick, you’ve started complex chemical reaction that will keep itself alive as long as the candle is intact and the flame is fed. before lighting the candle, make three qualitative observations and three quantitative observations of the candle. light is often the result of combustion or heating, or both. Fe +o2 → fe2o3 fe + o 2 → fe 2 o 3. Salt in water vs burning magnesium. color changes indicate chemical change.
Candlechangingstates
Is Lighting A Candle Chemical Change before lighting the candle, make three qualitative observations and three quantitative observations of the candle. A physical change, such as a state change or dissolving, does not create a new substance, but a chemical change does. When you light a candle wick, you’ve started complex chemical reaction that will keep itself alive as long as the candle is intact and the flame is fed. The wick absorbs the liquid wax, and it travels up the wick to the flame, where it combusts, keeping the flame going. before lighting the candle, make three qualitative observations and three quantitative observations of the candle. Salt in water vs burning magnesium. Fe +o2 → fe2o3 fe + o 2 → fe 2 o 3. Physical and chemical changes with examples. color changes indicate chemical change. Melting wax vs burning candle. all the light a candle makes comes from a chemical reaction known as combustion in which the wax (made from. lighting a candle. light is often the result of combustion or heating, or both. First, the heat from the flame melts the wax nearby.
From www.pinterest.com
Relight a Candle From a Distance With This Science Trick Candles Is Lighting A Candle Chemical Change Physical and chemical changes with examples. lighting a candle. When you light a candle wick, you’ve started complex chemical reaction that will keep itself alive as long as the candle is intact and the flame is fed. color changes indicate chemical change. A physical change, such as a state change or dissolving, does not create a new substance,. Is Lighting A Candle Chemical Change.
From exoyasbgc.blob.core.windows.net
How To Light A Candle Without A Holder at Julio Townsend blog Is Lighting A Candle Chemical Change Salt in water vs burning magnesium. before lighting the candle, make three qualitative observations and three quantitative observations of the candle. color changes indicate chemical change. Fe +o2 → fe2o3 fe + o 2 → fe 2 o 3. First, the heat from the flame melts the wax nearby. Melting wax vs burning candle. light is often. Is Lighting A Candle Chemical Change.
From www.thoughtco.com
Light a Candle with Smoke (Flame Science Trick) Is Lighting A Candle Chemical Change First, the heat from the flame melts the wax nearby. lighting a candle. light is often the result of combustion or heating, or both. Physical and chemical changes with examples. color changes indicate chemical change. A physical change, such as a state change or dissolving, does not create a new substance, but a chemical change does. Fe. Is Lighting A Candle Chemical Change.
From www.scienceabc.com
Why Does A Candle Only Produce Smoke When It's Extinguished? » ScienceABC Is Lighting A Candle Chemical Change First, the heat from the flame melts the wax nearby. color changes indicate chemical change. A physical change, such as a state change or dissolving, does not create a new substance, but a chemical change does. Salt in water vs burning magnesium. Physical and chemical changes with examples. light is often the result of combustion or heating, or. Is Lighting A Candle Chemical Change.
From www.slideserve.com
PPT Chemical Properties and Changes PowerPoint Presentation, free Is Lighting A Candle Chemical Change The wick absorbs the liquid wax, and it travels up the wick to the flame, where it combusts, keeping the flame going. all the light a candle makes comes from a chemical reaction known as combustion in which the wax (made from. A physical change, such as a state change or dissolving, does not create a new substance, but. Is Lighting A Candle Chemical Change.
From cetyhpps.blob.core.windows.net
Wax Candle Chemical Change at Frederick Ontiveros blog Is Lighting A Candle Chemical Change First, the heat from the flame melts the wax nearby. light is often the result of combustion or heating, or both. Fe +o2 → fe2o3 fe + o 2 → fe 2 o 3. color changes indicate chemical change. The wick absorbs the liquid wax, and it travels up the wick to the flame, where it combusts, keeping. Is Lighting A Candle Chemical Change.
From dxofkpuzk.blob.core.windows.net
Does Burning A Candle Absorb Or Release Energy at Tonya Staton blog Is Lighting A Candle Chemical Change color changes indicate chemical change. Salt in water vs burning magnesium. light is often the result of combustion or heating, or both. before lighting the candle, make three qualitative observations and three quantitative observations of the candle. all the light a candle makes comes from a chemical reaction known as combustion in which the wax (made. Is Lighting A Candle Chemical Change.
From studylib.net
Candlechangingstates Is Lighting A Candle Chemical Change A physical change, such as a state change or dissolving, does not create a new substance, but a chemical change does. Salt in water vs burning magnesium. color changes indicate chemical change. The wick absorbs the liquid wax, and it travels up the wick to the flame, where it combusts, keeping the flame going. all the light a. Is Lighting A Candle Chemical Change.
From www.slideserve.com
PPT Physical or Chemical Change? PowerPoint Presentation, free Is Lighting A Candle Chemical Change Salt in water vs burning magnesium. color changes indicate chemical change. lighting a candle. Physical and chemical changes with examples. all the light a candle makes comes from a chemical reaction known as combustion in which the wax (made from. before lighting the candle, make three qualitative observations and three quantitative observations of the candle. When. Is Lighting A Candle Chemical Change.
From www.slideserve.com
PPT Physical and Chemical Changes PowerPoint Presentation, free Is Lighting A Candle Chemical Change The wick absorbs the liquid wax, and it travels up the wick to the flame, where it combusts, keeping the flame going. color changes indicate chemical change. all the light a candle makes comes from a chemical reaction known as combustion in which the wax (made from. lighting a candle. Melting wax vs burning candle. A physical. Is Lighting A Candle Chemical Change.
From www.iammarriamhere.com
Three ways to lighting a candle..Be the Light of Life. Is Lighting A Candle Chemical Change all the light a candle makes comes from a chemical reaction known as combustion in which the wax (made from. When you light a candle wick, you’ve started complex chemical reaction that will keep itself alive as long as the candle is intact and the flame is fed. A physical change, such as a state change or dissolving, does. Is Lighting A Candle Chemical Change.
From www.youtube.com
[JEE ADVANCED] HEAT CONDUCTION IN A BURNING CANDLE [ ADVANCED PROBLEMS Is Lighting A Candle Chemical Change Salt in water vs burning magnesium. A physical change, such as a state change or dissolving, does not create a new substance, but a chemical change does. before lighting the candle, make three qualitative observations and three quantitative observations of the candle. Fe +o2 → fe2o3 fe + o 2 → fe 2 o 3. Melting wax vs burning. Is Lighting A Candle Chemical Change.
From www.slideshare.net
light a candle science task Is Lighting A Candle Chemical Change First, the heat from the flame melts the wax nearby. Melting wax vs burning candle. before lighting the candle, make three qualitative observations and three quantitative observations of the candle. The wick absorbs the liquid wax, and it travels up the wick to the flame, where it combusts, keeping the flame going. When you light a candle wick, you’ve. Is Lighting A Candle Chemical Change.
From www.youtube.com
Chemical Science Experiment! How To Make Rainbow Coloured Flame Is Lighting A Candle Chemical Change light is often the result of combustion or heating, or both. A physical change, such as a state change or dissolving, does not create a new substance, but a chemical change does. First, the heat from the flame melts the wax nearby. before lighting the candle, make three qualitative observations and three quantitative observations of the candle. Fe. Is Lighting A Candle Chemical Change.
From www.chemistryviews.org
The Science Behind Candles ChemistryViews Is Lighting A Candle Chemical Change The wick absorbs the liquid wax, and it travels up the wick to the flame, where it combusts, keeping the flame going. light is often the result of combustion or heating, or both. Physical and chemical changes with examples. lighting a candle. all the light a candle makes comes from a chemical reaction known as combustion in. Is Lighting A Candle Chemical Change.
From www.youtube.com
Burning Candle Chemical Reaction YouTube Is Lighting A Candle Chemical Change before lighting the candle, make three qualitative observations and three quantitative observations of the candle. A physical change, such as a state change or dissolving, does not create a new substance, but a chemical change does. The wick absorbs the liquid wax, and it travels up the wick to the flame, where it combusts, keeping the flame going. Melting. Is Lighting A Candle Chemical Change.
From inchemistry.acs.org
Shining a Light on Candles inChemistry Is Lighting A Candle Chemical Change color changes indicate chemical change. Fe +o2 → fe2o3 fe + o 2 → fe 2 o 3. all the light a candle makes comes from a chemical reaction known as combustion in which the wax (made from. Salt in water vs burning magnesium. First, the heat from the flame melts the wax nearby. A physical change, such. Is Lighting A Candle Chemical Change.
From cocoapuffs321.blogspot.com
cocoapuffs321 Physical and Chemical Changes Is Lighting A Candle Chemical Change The wick absorbs the liquid wax, and it travels up the wick to the flame, where it combusts, keeping the flame going. First, the heat from the flame melts the wax nearby. A physical change, such as a state change or dissolving, does not create a new substance, but a chemical change does. light is often the result of. Is Lighting A Candle Chemical Change.
From www.alamy.com
Hand of person heating chemical substance in spoon on candle light Is Lighting A Candle Chemical Change Salt in water vs burning magnesium. The wick absorbs the liquid wax, and it travels up the wick to the flame, where it combusts, keeping the flame going. When you light a candle wick, you’ve started complex chemical reaction that will keep itself alive as long as the candle is intact and the flame is fed. First, the heat from. Is Lighting A Candle Chemical Change.
From www.lovetoknow.com
How to Light a Candle Without a Lighter 5 Simple Hacks LoveToKnow Is Lighting A Candle Chemical Change before lighting the candle, make three qualitative observations and three quantitative observations of the candle. Salt in water vs burning magnesium. all the light a candle makes comes from a chemical reaction known as combustion in which the wax (made from. First, the heat from the flame melts the wax nearby. light is often the result of. Is Lighting A Candle Chemical Change.
From www.cushyfamily.com
Is Lighting A Candle A Chemical Change? (Helpful Examples) Is Lighting A Candle Chemical Change Salt in water vs burning magnesium. A physical change, such as a state change or dissolving, does not create a new substance, but a chemical change does. Physical and chemical changes with examples. Melting wax vs burning candle. The wick absorbs the liquid wax, and it travels up the wick to the flame, where it combusts, keeping the flame going.. Is Lighting A Candle Chemical Change.
From www.youtube.com
IS BURNING OF CANDLE A CHEMICAL CHANGE OR A PHYSICAL CHANGE? YouTube Is Lighting A Candle Chemical Change First, the heat from the flame melts the wax nearby. A physical change, such as a state change or dissolving, does not create a new substance, but a chemical change does. Melting wax vs burning candle. lighting a candle. light is often the result of combustion or heating, or both. before lighting the candle, make three qualitative. Is Lighting A Candle Chemical Change.
From www.slideserve.com
PPT Chemical and Physical changes PowerPoint Presentation, free Is Lighting A Candle Chemical Change Fe +o2 → fe2o3 fe + o 2 → fe 2 o 3. all the light a candle makes comes from a chemical reaction known as combustion in which the wax (made from. First, the heat from the flame melts the wax nearby. before lighting the candle, make three qualitative observations and three quantitative observations of the candle.. Is Lighting A Candle Chemical Change.
From jaysonkruwfitzgerald.blogspot.com
Is Lighting a Candle Physical or Chemical JaysonkruwFitzgerald Is Lighting A Candle Chemical Change Salt in water vs burning magnesium. The wick absorbs the liquid wax, and it travels up the wick to the flame, where it combusts, keeping the flame going. A physical change, such as a state change or dissolving, does not create a new substance, but a chemical change does. First, the heat from the flame melts the wax nearby. . Is Lighting A Candle Chemical Change.
From www.cushyfamily.com
Is Lighting A Candle Physical Or Chemical? Answer) Is Lighting A Candle Chemical Change Salt in water vs burning magnesium. Melting wax vs burning candle. A physical change, such as a state change or dissolving, does not create a new substance, but a chemical change does. lighting a candle. Physical and chemical changes with examples. color changes indicate chemical change. First, the heat from the flame melts the wax nearby. light. Is Lighting A Candle Chemical Change.
From www.youtube.com
Vacuum Candle Experiment YouTube Is Lighting A Candle Chemical Change Melting wax vs burning candle. When you light a candle wick, you’ve started complex chemical reaction that will keep itself alive as long as the candle is intact and the flame is fed. lighting a candle. The wick absorbs the liquid wax, and it travels up the wick to the flame, where it combusts, keeping the flame going. . Is Lighting A Candle Chemical Change.
From studylib.net
Burning Candle Activity Is Lighting A Candle Chemical Change Salt in water vs burning magnesium. The wick absorbs the liquid wax, and it travels up the wick to the flame, where it combusts, keeping the flame going. Fe +o2 → fe2o3 fe + o 2 → fe 2 o 3. all the light a candle makes comes from a chemical reaction known as combustion in which the wax. Is Lighting A Candle Chemical Change.
From haikudeck.com
Chemical And Physical Changes by Julia Sheldon Is Lighting A Candle Chemical Change Melting wax vs burning candle. Fe +o2 → fe2o3 fe + o 2 → fe 2 o 3. When you light a candle wick, you’ve started complex chemical reaction that will keep itself alive as long as the candle is intact and the flame is fed. Physical and chemical changes with examples. light is often the result of combustion. Is Lighting A Candle Chemical Change.
From www.youtube.com
Candle oxygen experiment YouTube Is Lighting A Candle Chemical Change before lighting the candle, make three qualitative observations and three quantitative observations of the candle. Melting wax vs burning candle. When you light a candle wick, you’ve started complex chemical reaction that will keep itself alive as long as the candle is intact and the flame is fed. light is often the result of combustion or heating, or. Is Lighting A Candle Chemical Change.
From www.teachoo.com
Which of the following does not involve a chemical reaction? Teachoo Is Lighting A Candle Chemical Change Salt in water vs burning magnesium. Melting wax vs burning candle. The wick absorbs the liquid wax, and it travels up the wick to the flame, where it combusts, keeping the flame going. Physical and chemical changes with examples. light is often the result of combustion or heating, or both. First, the heat from the flame melts the wax. Is Lighting A Candle Chemical Change.
From www.pinterest.com
Do You Know What Toxins Are In Your Candles? Favorite candles Is Lighting A Candle Chemical Change Physical and chemical changes with examples. all the light a candle makes comes from a chemical reaction known as combustion in which the wax (made from. Salt in water vs burning magnesium. lighting a candle. Fe +o2 → fe2o3 fe + o 2 → fe 2 o 3. The wick absorbs the liquid wax, and it travels up. Is Lighting A Candle Chemical Change.
From www.youtube.com
Lighting a candle without touching its wick Toppr Experiments YouTube Is Lighting A Candle Chemical Change A physical change, such as a state change or dissolving, does not create a new substance, but a chemical change does. When you light a candle wick, you’ve started complex chemical reaction that will keep itself alive as long as the candle is intact and the flame is fed. color changes indicate chemical change. First, the heat from the. Is Lighting A Candle Chemical Change.
From vhmsscience8.weebly.com
Candle Lab VISTA HEIGHTS 8TH GRADE SCIENCE Is Lighting A Candle Chemical Change First, the heat from the flame melts the wax nearby. before lighting the candle, make three qualitative observations and three quantitative observations of the candle. Physical and chemical changes with examples. lighting a candle. The wick absorbs the liquid wax, and it travels up the wick to the flame, where it combusts, keeping the flame going. A physical. Is Lighting A Candle Chemical Change.
From www.thoughtco.com
An Introduction to Combustion (Burning) Reactions Is Lighting A Candle Chemical Change Salt in water vs burning magnesium. Physical and chemical changes with examples. Melting wax vs burning candle. First, the heat from the flame melts the wax nearby. before lighting the candle, make three qualitative observations and three quantitative observations of the candle. Fe +o2 → fe2o3 fe + o 2 → fe 2 o 3. lighting a candle.. Is Lighting A Candle Chemical Change.
From blog.marylandmatch.com
How to Light a Candle Properly Is Lighting A Candle Chemical Change The wick absorbs the liquid wax, and it travels up the wick to the flame, where it combusts, keeping the flame going. all the light a candle makes comes from a chemical reaction known as combustion in which the wax (made from. color changes indicate chemical change. light is often the result of combustion or heating, or. Is Lighting A Candle Chemical Change.