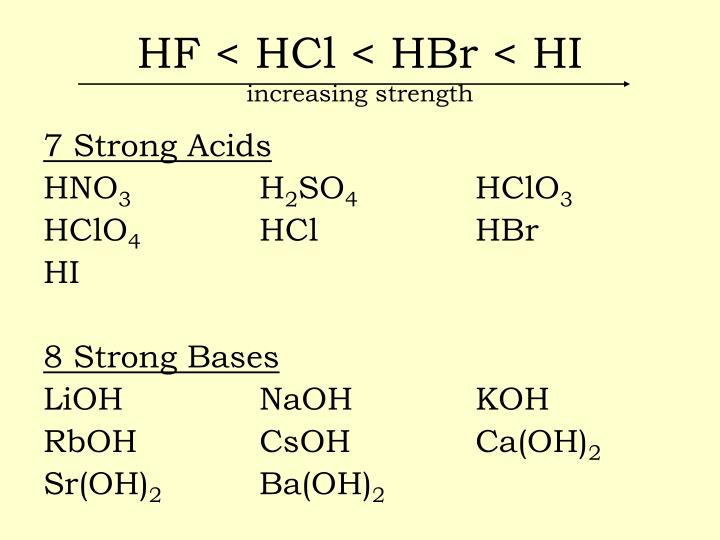
Is Oh A Strong Acid . The table lists the k a values and the strength of each acid and base. Any acid that dissociates 100% into ions is called a strong acid. Hc 2 h 3 o 2 is an example of a. A base that is a strong electrolyte is called a strong base, while a. 34 rows read these instructions to learn how to use this acids and bases chart. In this tutorial, you will learn about the properties and ph of strong acids and bases, and how to calculate their ph, poh, pka, and. As one can see, most of the strong bases are hydroxides of alkali metals or alkaline earth metals. An acid that is a strong electrolyte is called a strong acid, while an acid that is a weak electrolyte is a weak acid. If it does not dissociate 100%, it is a weak acid. A weak acid is one. Strong acids are acids that are completely or nearly 100% ionized in their solutions; A strong acid is one that loses h + easily, meaning that its conjugate base holds the h + weakly and is therefore a weak base.
from www.slideserve.com
If it does not dissociate 100%, it is a weak acid. In this tutorial, you will learn about the properties and ph of strong acids and bases, and how to calculate their ph, poh, pka, and. Any acid that dissociates 100% into ions is called a strong acid. A strong acid is one that loses h + easily, meaning that its conjugate base holds the h + weakly and is therefore a weak base. A weak acid is one. A base that is a strong electrolyte is called a strong base, while a. Hc 2 h 3 o 2 is an example of a. 34 rows read these instructions to learn how to use this acids and bases chart. Strong acids are acids that are completely or nearly 100% ionized in their solutions; As one can see, most of the strong bases are hydroxides of alkali metals or alkaline earth metals.
PPT Acids & Bases PowerPoint Presentation ID6155898
Is Oh A Strong Acid As one can see, most of the strong bases are hydroxides of alkali metals or alkaline earth metals. As one can see, most of the strong bases are hydroxides of alkali metals or alkaline earth metals. A weak acid is one. In this tutorial, you will learn about the properties and ph of strong acids and bases, and how to calculate their ph, poh, pka, and. The table lists the k a values and the strength of each acid and base. A strong acid is one that loses h + easily, meaning that its conjugate base holds the h + weakly and is therefore a weak base. A base that is a strong electrolyte is called a strong base, while a. If it does not dissociate 100%, it is a weak acid. Any acid that dissociates 100% into ions is called a strong acid. An acid that is a strong electrolyte is called a strong acid, while an acid that is a weak electrolyte is a weak acid. Hc 2 h 3 o 2 is an example of a. Strong acids are acids that are completely or nearly 100% ionized in their solutions; 34 rows read these instructions to learn how to use this acids and bases chart.
From learningmagicproffered.z21.web.core.windows.net
Nomenclature Of Acids And Bases Is Oh A Strong Acid Strong acids are acids that are completely or nearly 100% ionized in their solutions; As one can see, most of the strong bases are hydroxides of alkali metals or alkaline earth metals. A base that is a strong electrolyte is called a strong base, while a. Any acid that dissociates 100% into ions is called a strong acid. In this. Is Oh A Strong Acid.
From praxilabs.com
Learn All About The Strong Acids and Bases PraxiLabs Is Oh A Strong Acid If it does not dissociate 100%, it is a weak acid. An acid that is a strong electrolyte is called a strong acid, while an acid that is a weak electrolyte is a weak acid. A weak acid is one. As one can see, most of the strong bases are hydroxides of alkali metals or alkaline earth metals. Strong acids. Is Oh A Strong Acid.
From www.slideserve.com
PPT Chapter 15 Acids and Bases PowerPoint Presentation, free download Is Oh A Strong Acid 34 rows read these instructions to learn how to use this acids and bases chart. If it does not dissociate 100%, it is a weak acid. Strong acids are acids that are completely or nearly 100% ionized in their solutions; The table lists the k a values and the strength of each acid and base. A weak acid is one.. Is Oh A Strong Acid.
From www.chegg.com
Solved Part A Which compound is a stronger acid? OH OH Is Oh A Strong Acid A weak acid is one. If it does not dissociate 100%, it is a weak acid. Hc 2 h 3 o 2 is an example of a. An acid that is a strong electrolyte is called a strong acid, while an acid that is a weak electrolyte is a weak acid. The table lists the k a values and the. Is Oh A Strong Acid.
From blog.praxilabs.com
Learn All About The Strong Acids and Bases PraxiLabs Is Oh A Strong Acid If it does not dissociate 100%, it is a weak acid. Strong acids are acids that are completely or nearly 100% ionized in their solutions; In this tutorial, you will learn about the properties and ph of strong acids and bases, and how to calculate their ph, poh, pka, and. A weak acid is one. Hc 2 h 3 o. Is Oh A Strong Acid.
From www.thesciencehive.co.uk
Acids* — the science sauce Is Oh A Strong Acid A base that is a strong electrolyte is called a strong base, while a. Any acid that dissociates 100% into ions is called a strong acid. The table lists the k a values and the strength of each acid and base. As one can see, most of the strong bases are hydroxides of alkali metals or alkaline earth metals. Strong. Is Oh A Strong Acid.
From www.thoughtco.com
List of Common Strong and Weak Acids Is Oh A Strong Acid In this tutorial, you will learn about the properties and ph of strong acids and bases, and how to calculate their ph, poh, pka, and. If it does not dissociate 100%, it is a weak acid. 34 rows read these instructions to learn how to use this acids and bases chart. A base that is a strong electrolyte is called. Is Oh A Strong Acid.
From www.youtube.com
Acid Base Strength Which Is Stronger? YouTube Is Oh A Strong Acid Strong acids are acids that are completely or nearly 100% ionized in their solutions; Any acid that dissociates 100% into ions is called a strong acid. The table lists the k a values and the strength of each acid and base. In this tutorial, you will learn about the properties and ph of strong acids and bases, and how to. Is Oh A Strong Acid.
From ar.inspiredpencil.com
Ch3 Ch3 Acid Base Or Salt Is Oh A Strong Acid 34 rows read these instructions to learn how to use this acids and bases chart. A weak acid is one. If it does not dissociate 100%, it is a weak acid. Any acid that dissociates 100% into ions is called a strong acid. As one can see, most of the strong bases are hydroxides of alkali metals or alkaline earth. Is Oh A Strong Acid.
From www.teachoo.com
List of Strong Bases 7+ Examples Chemistry Teachoo Is Oh A Strong Acid A strong acid is one that loses h + easily, meaning that its conjugate base holds the h + weakly and is therefore a weak base. 34 rows read these instructions to learn how to use this acids and bases chart. If it does not dissociate 100%, it is a weak acid. As one can see, most of the strong. Is Oh A Strong Acid.
From brainly.com
Use the following table to answer the question. Strong acids Weak acids Is Oh A Strong Acid In this tutorial, you will learn about the properties and ph of strong acids and bases, and how to calculate their ph, poh, pka, and. As one can see, most of the strong bases are hydroxides of alkali metals or alkaline earth metals. A weak acid is one. The table lists the k a values and the strength of each. Is Oh A Strong Acid.
From med.libretexts.org
2.4 Acids and Bases Medicine LibreTexts Is Oh A Strong Acid If it does not dissociate 100%, it is a weak acid. Strong acids are acids that are completely or nearly 100% ionized in their solutions; Hc 2 h 3 o 2 is an example of a. The table lists the k a values and the strength of each acid and base. A weak acid is one. An acid that is. Is Oh A Strong Acid.
From www.youtube.com
Is Ca(OH)2 an acid or base? YouTube Is Oh A Strong Acid As one can see, most of the strong bases are hydroxides of alkali metals or alkaline earth metals. If it does not dissociate 100%, it is a weak acid. The table lists the k a values and the strength of each acid and base. An acid that is a strong electrolyte is called a strong acid, while an acid that. Is Oh A Strong Acid.
From sciencewithmrsb.weebly.com
Acids and Bases Science with Mrs Beggs Is Oh A Strong Acid The table lists the k a values and the strength of each acid and base. 34 rows read these instructions to learn how to use this acids and bases chart. As one can see, most of the strong bases are hydroxides of alkali metals or alkaline earth metals. In this tutorial, you will learn about the properties and ph of. Is Oh A Strong Acid.
From www.slideserve.com
PPT MODERN CHEMISTRY CHAPTER 14 ACIDS AND BASES PowerPoint Is Oh A Strong Acid A weak acid is one. An acid that is a strong electrolyte is called a strong acid, while an acid that is a weak electrolyte is a weak acid. A base that is a strong electrolyte is called a strong base, while a. As one can see, most of the strong bases are hydroxides of alkali metals or alkaline earth. Is Oh A Strong Acid.
From www.expii.com
Strong and Weak Acids and Bases — Definition & Examples Expii Is Oh A Strong Acid An acid that is a strong electrolyte is called a strong acid, while an acid that is a weak electrolyte is a weak acid. If it does not dissociate 100%, it is a weak acid. A weak acid is one. 34 rows read these instructions to learn how to use this acids and bases chart. Any acid that dissociates 100%. Is Oh A Strong Acid.
From www.youtube.com
How To Memorize The Strong Acids and Strong Bases YouTube Is Oh A Strong Acid An acid that is a strong electrolyte is called a strong acid, while an acid that is a weak electrolyte is a weak acid. Strong acids are acids that are completely or nearly 100% ionized in their solutions; A weak acid is one. A base that is a strong electrolyte is called a strong base, while a. Any acid that. Is Oh A Strong Acid.
From www.dreamstime.com
Strong and Weak Acids Collection with Educational Diagram Outline Is Oh A Strong Acid Any acid that dissociates 100% into ions is called a strong acid. 34 rows read these instructions to learn how to use this acids and bases chart. Strong acids are acids that are completely or nearly 100% ionized in their solutions; If it does not dissociate 100%, it is a weak acid. A weak acid is one. In this tutorial,. Is Oh A Strong Acid.
From brainly.in
explain the term strong acids,weak acids , strong base and weak bases Is Oh A Strong Acid If it does not dissociate 100%, it is a weak acid. An acid that is a strong electrolyte is called a strong acid, while an acid that is a weak electrolyte is a weak acid. Any acid that dissociates 100% into ions is called a strong acid. As one can see, most of the strong bases are hydroxides of alkali. Is Oh A Strong Acid.
From www.slideserve.com
PPT AcidBase Reactions PowerPoint Presentation, free download ID Is Oh A Strong Acid An acid that is a strong electrolyte is called a strong acid, while an acid that is a weak electrolyte is a weak acid. As one can see, most of the strong bases are hydroxides of alkali metals or alkaline earth metals. 34 rows read these instructions to learn how to use this acids and bases chart. A strong acid. Is Oh A Strong Acid.
From www.slideserve.com
PPT Strong and Weak Acids and Bases PowerPoint Presentation, free Is Oh A Strong Acid The table lists the k a values and the strength of each acid and base. Hc 2 h 3 o 2 is an example of a. A weak acid is one. In this tutorial, you will learn about the properties and ph of strong acids and bases, and how to calculate their ph, poh, pka, and. Any acid that dissociates. Is Oh A Strong Acid.
From www.slideshare.net
Lect w7 152_abbrev_ intro to acids and bases_alg Is Oh A Strong Acid The table lists the k a values and the strength of each acid and base. An acid that is a strong electrolyte is called a strong acid, while an acid that is a weak electrolyte is a weak acid. Strong acids are acids that are completely or nearly 100% ionized in their solutions; As one can see, most of the. Is Oh A Strong Acid.
From www.expii.com
Strong and Weak Acids and Bases — Definition & Examples Expii Is Oh A Strong Acid The table lists the k a values and the strength of each acid and base. A strong acid is one that loses h + easily, meaning that its conjugate base holds the h + weakly and is therefore a weak base. A base that is a strong electrolyte is called a strong base, while a. Strong acids are acids that. Is Oh A Strong Acid.
From www.teachoo.com
Examples of Weak Base (5 Examples with Images) Teachoo Chemistry Is Oh A Strong Acid 34 rows read these instructions to learn how to use this acids and bases chart. The table lists the k a values and the strength of each acid and base. A base that is a strong electrolyte is called a strong base, while a. Strong acids are acids that are completely or nearly 100% ionized in their solutions; An acid. Is Oh A Strong Acid.
From www.teachoo.com
Examples of Weak Acids 5+ Examples Teachoo Teachoo Questions Is Oh A Strong Acid Any acid that dissociates 100% into ions is called a strong acid. A base that is a strong electrolyte is called a strong base, while a. Hc 2 h 3 o 2 is an example of a. A weak acid is one. A strong acid is one that loses h + easily, meaning that its conjugate base holds the h. Is Oh A Strong Acid.
From www.numerade.com
SOLVED Classify these compounds by whether they behave as strong acids Is Oh A Strong Acid Strong acids are acids that are completely or nearly 100% ionized in their solutions; Any acid that dissociates 100% into ions is called a strong acid. As one can see, most of the strong bases are hydroxides of alkali metals or alkaline earth metals. A weak acid is one. 34 rows read these instructions to learn how to use this. Is Oh A Strong Acid.
From techiescientist.com
pH of Lactic Acid — Strong or Weak? Techiescientist Is Oh A Strong Acid An acid that is a strong electrolyte is called a strong acid, while an acid that is a weak electrolyte is a weak acid. If it does not dissociate 100%, it is a weak acid. A base that is a strong electrolyte is called a strong base, while a. Strong acids are acids that are completely or nearly 100% ionized. Is Oh A Strong Acid.
From www.teachoo.com
List of Strong Acids (More than 8 examples, with images) Teachoo Is Oh A Strong Acid As one can see, most of the strong bases are hydroxides of alkali metals or alkaline earth metals. Any acid that dissociates 100% into ions is called a strong acid. An acid that is a strong electrolyte is called a strong acid, while an acid that is a weak electrolyte is a weak acid. If it does not dissociate 100%,. Is Oh A Strong Acid.
From es.slideshare.net
1 21 What Acids & Bases Part Ii Is Oh A Strong Acid Strong acids are acids that are completely or nearly 100% ionized in their solutions; If it does not dissociate 100%, it is a weak acid. In this tutorial, you will learn about the properties and ph of strong acids and bases, and how to calculate their ph, poh, pka, and. A strong acid is one that loses h + easily,. Is Oh A Strong Acid.
From www.youtube.com
Weak acidstrong base reactions Acids and bases AP Chemistry Khan Is Oh A Strong Acid Hc 2 h 3 o 2 is an example of a. As one can see, most of the strong bases are hydroxides of alkali metals or alkaline earth metals. A weak acid is one. The table lists the k a values and the strength of each acid and base. A strong acid is one that loses h + easily, meaning. Is Oh A Strong Acid.
From www.slideserve.com
PPT Acids & Bases PowerPoint Presentation ID6155898 Is Oh A Strong Acid Hc 2 h 3 o 2 is an example of a. 34 rows read these instructions to learn how to use this acids and bases chart. A strong acid is one that loses h + easily, meaning that its conjugate base holds the h + weakly and is therefore a weak base. An acid that is a strong electrolyte is. Is Oh A Strong Acid.
From www.slideserve.com
PPT The Chemistry of Acids and Bases PowerPoint Presentation, free Is Oh A Strong Acid Strong acids are acids that are completely or nearly 100% ionized in their solutions; Any acid that dissociates 100% into ions is called a strong acid. A strong acid is one that loses h + easily, meaning that its conjugate base holds the h + weakly and is therefore a weak base. A weak acid is one. The table lists. Is Oh A Strong Acid.
From www.revision.my
6.3.1 Strength of Acids and Alkalis Revision.my Is Oh A Strong Acid Any acid that dissociates 100% into ions is called a strong acid. In this tutorial, you will learn about the properties and ph of strong acids and bases, and how to calculate their ph, poh, pka, and. A weak acid is one. Strong acids are acids that are completely or nearly 100% ionized in their solutions; If it does not. Is Oh A Strong Acid.
From www.slideserve.com
PPT Common Strong acids PowerPoint Presentation, free download ID Is Oh A Strong Acid The table lists the k a values and the strength of each acid and base. As one can see, most of the strong bases are hydroxides of alkali metals or alkaline earth metals. Hc 2 h 3 o 2 is an example of a. In this tutorial, you will learn about the properties and ph of strong acids and bases,. Is Oh A Strong Acid.
From slideplayer.com
Strong Acid/Base Calculations ppt download Is Oh A Strong Acid 34 rows read these instructions to learn how to use this acids and bases chart. A base that is a strong electrolyte is called a strong base, while a. A weak acid is one. If it does not dissociate 100%, it is a weak acid. As one can see, most of the strong bases are hydroxides of alkali metals or. Is Oh A Strong Acid.