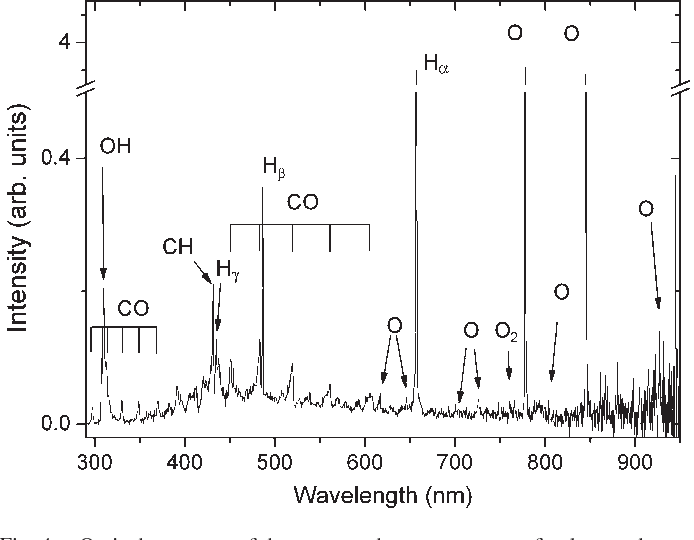
Spectroscopy Emission Oxygen . 60 a diode laser was. The emission spectra of various atoms. Oxygen was also sensed directly by employing gas correlation absorption spectroscopy using multimode diode lasers. Emission and absorption spectra form the basis of spectroscopy, which uses spectra to provide information about the structure and the composition of a substance or an object. When hydrogen gas is placed into a tube and. The three atomic emission spectra for oxygen can be shown on graph bellow: An analyte in an excited state possesses an energy, e2, that is greater than its energy when it is in a lower energy state, e1. Strong lines of oxygen ( o ) strong lines of oxygen ( o ) intensity. The emission spectrum (or line spectrum) of a chemical element is the unique pattern of light obtained when the element is subjected to heat or electricity.
from www.semanticscholar.org
60 a diode laser was. When hydrogen gas is placed into a tube and. An analyte in an excited state possesses an energy, e2, that is greater than its energy when it is in a lower energy state, e1. Strong lines of oxygen ( o ) strong lines of oxygen ( o ) intensity. The three atomic emission spectra for oxygen can be shown on graph bellow: The emission spectrum (or line spectrum) of a chemical element is the unique pattern of light obtained when the element is subjected to heat or electricity. Oxygen was also sensed directly by employing gas correlation absorption spectroscopy using multimode diode lasers. Emission and absorption spectra form the basis of spectroscopy, which uses spectra to provide information about the structure and the composition of a substance or an object. The emission spectra of various atoms.
Figure 4 from Monitoring Oxygen Plasma Treatment of Polypropylene With
Spectroscopy Emission Oxygen The emission spectra of various atoms. The emission spectrum (or line spectrum) of a chemical element is the unique pattern of light obtained when the element is subjected to heat or electricity. Strong lines of oxygen ( o ) strong lines of oxygen ( o ) intensity. 60 a diode laser was. An analyte in an excited state possesses an energy, e2, that is greater than its energy when it is in a lower energy state, e1. Emission and absorption spectra form the basis of spectroscopy, which uses spectra to provide information about the structure and the composition of a substance or an object. Oxygen was also sensed directly by employing gas correlation absorption spectroscopy using multimode diode lasers. The emission spectra of various atoms. The three atomic emission spectra for oxygen can be shown on graph bellow: When hydrogen gas is placed into a tube and.
From www.researchgate.net
(PDF) Optical emission spectroscopy of oxygen plasma induced by IR CO2 Spectroscopy Emission Oxygen An analyte in an excited state possesses an energy, e2, that is greater than its energy when it is in a lower energy state, e1. Emission and absorption spectra form the basis of spectroscopy, which uses spectra to provide information about the structure and the composition of a substance or an object. The three atomic emission spectra for oxygen can. Spectroscopy Emission Oxygen.
From www.thoughtco.com
What Is Luminosity and What does it Tell Us? Spectroscopy Emission Oxygen An analyte in an excited state possesses an energy, e2, that is greater than its energy when it is in a lower energy state, e1. When hydrogen gas is placed into a tube and. The emission spectrum (or line spectrum) of a chemical element is the unique pattern of light obtained when the element is subjected to heat or electricity.. Spectroscopy Emission Oxygen.
From www.researchgate.net
Optical emission spectra of O2, N2, Ar, and Air feeding gases plasma Spectroscopy Emission Oxygen An analyte in an excited state possesses an energy, e2, that is greater than its energy when it is in a lower energy state, e1. 60 a diode laser was. Emission and absorption spectra form the basis of spectroscopy, which uses spectra to provide information about the structure and the composition of a substance or an object. When hydrogen gas. Spectroscopy Emission Oxygen.
From www.researchgate.net
Emission spectra of singlet oxygen generated by COTP and CO T NPs in Spectroscopy Emission Oxygen Emission and absorption spectra form the basis of spectroscopy, which uses spectra to provide information about the structure and the composition of a substance or an object. Strong lines of oxygen ( o ) strong lines of oxygen ( o ) intensity. An analyte in an excited state possesses an energy, e2, that is greater than its energy when it. Spectroscopy Emission Oxygen.
From www.researchgate.net
Optical emission spectrum of heliumoxygen (He/O2) plasma established Spectroscopy Emission Oxygen Oxygen was also sensed directly by employing gas correlation absorption spectroscopy using multimode diode lasers. 60 a diode laser was. The emission spectrum (or line spectrum) of a chemical element is the unique pattern of light obtained when the element is subjected to heat or electricity. The emission spectra of various atoms. An analyte in an excited state possesses an. Spectroscopy Emission Oxygen.
From exyghgyge.blob.core.windows.net
Types Of Optical Emission Spectroscopy at Mary Maclean blog Spectroscopy Emission Oxygen The three atomic emission spectra for oxygen can be shown on graph bellow: Emission and absorption spectra form the basis of spectroscopy, which uses spectra to provide information about the structure and the composition of a substance or an object. Oxygen was also sensed directly by employing gas correlation absorption spectroscopy using multimode diode lasers. The emission spectrum (or line. Spectroscopy Emission Oxygen.
From www.researchgate.net
Photoluminescence emission spectra of oxygen vacancies in asprepared Spectroscopy Emission Oxygen The emission spectrum (or line spectrum) of a chemical element is the unique pattern of light obtained when the element is subjected to heat or electricity. 60 a diode laser was. The emission spectra of various atoms. Oxygen was also sensed directly by employing gas correlation absorption spectroscopy using multimode diode lasers. An analyte in an excited state possesses an. Spectroscopy Emission Oxygen.
From jascoinc.com
Detection of Phosphorescence from Singlet Oxygen using NIR Spectroscopy Emission Oxygen Strong lines of oxygen ( o ) strong lines of oxygen ( o ) intensity. When hydrogen gas is placed into a tube and. Emission and absorption spectra form the basis of spectroscopy, which uses spectra to provide information about the structure and the composition of a substance or an object. 60 a diode laser was. The emission spectrum (or. Spectroscopy Emission Oxygen.
From www.slideserve.com
PPT Chapter 7 The QuantumMechanical Model of the Atom PowerPoint Spectroscopy Emission Oxygen 60 a diode laser was. The emission spectra of various atoms. The emission spectrum (or line spectrum) of a chemical element is the unique pattern of light obtained when the element is subjected to heat or electricity. Strong lines of oxygen ( o ) strong lines of oxygen ( o ) intensity. When hydrogen gas is placed into a tube. Spectroscopy Emission Oxygen.
From www.researchgate.net
The recorded optical emission spectrum at different oxygen percentage Spectroscopy Emission Oxygen 60 a diode laser was. An analyte in an excited state possesses an energy, e2, that is greater than its energy when it is in a lower energy state, e1. The emission spectrum (or line spectrum) of a chemical element is the unique pattern of light obtained when the element is subjected to heat or electricity. Oxygen was also sensed. Spectroscopy Emission Oxygen.
From www.researchgate.net
Optical emission spectrum of RF nitrogen plasma and its active species Spectroscopy Emission Oxygen Strong lines of oxygen ( o ) strong lines of oxygen ( o ) intensity. Emission and absorption spectra form the basis of spectroscopy, which uses spectra to provide information about the structure and the composition of a substance or an object. The emission spectra of various atoms. Oxygen was also sensed directly by employing gas correlation absorption spectroscopy using. Spectroscopy Emission Oxygen.
From joihsprvv.blob.core.windows.net
Emission Spectra Bbc Bitesize at Jose Doty blog Spectroscopy Emission Oxygen Oxygen was also sensed directly by employing gas correlation absorption spectroscopy using multimode diode lasers. The three atomic emission spectra for oxygen can be shown on graph bellow: The emission spectrum (or line spectrum) of a chemical element is the unique pattern of light obtained when the element is subjected to heat or electricity. Strong lines of oxygen ( o. Spectroscopy Emission Oxygen.
From ar.inspiredpencil.com
Nitrogen Emission Spectrum Wavelengths Spectroscopy Emission Oxygen The emission spectra of various atoms. When hydrogen gas is placed into a tube and. The three atomic emission spectra for oxygen can be shown on graph bellow: Strong lines of oxygen ( o ) strong lines of oxygen ( o ) intensity. Oxygen was also sensed directly by employing gas correlation absorption spectroscopy using multimode diode lasers. An analyte. Spectroscopy Emission Oxygen.
From www.researchgate.net
(a) Emission spectrum of oxygen at 0.95 Torr, 35 mA, 1800 V recorded Spectroscopy Emission Oxygen Oxygen was also sensed directly by employing gas correlation absorption spectroscopy using multimode diode lasers. Emission and absorption spectra form the basis of spectroscopy, which uses spectra to provide information about the structure and the composition of a substance or an object. An analyte in an excited state possesses an energy, e2, that is greater than its energy when it. Spectroscopy Emission Oxygen.
From www.researchgate.net
(Color online) Typical optical emission spectra of reactive chemical Spectroscopy Emission Oxygen Strong lines of oxygen ( o ) strong lines of oxygen ( o ) intensity. When hydrogen gas is placed into a tube and. Emission and absorption spectra form the basis of spectroscopy, which uses spectra to provide information about the structure and the composition of a substance or an object. 60 a diode laser was. The emission spectra of. Spectroscopy Emission Oxygen.
From chem.libretexts.org
7.3 The Atomic Spectrum of Hydrogen Chemistry LibreTexts Spectroscopy Emission Oxygen Oxygen was also sensed directly by employing gas correlation absorption spectroscopy using multimode diode lasers. An analyte in an excited state possesses an energy, e2, that is greater than its energy when it is in a lower energy state, e1. When hydrogen gas is placed into a tube and. Strong lines of oxygen ( o ) strong lines of oxygen. Spectroscopy Emission Oxygen.
From www.semanticscholar.org
Figure 4 from Monitoring Oxygen Plasma Treatment of Polypropylene With Spectroscopy Emission Oxygen When hydrogen gas is placed into a tube and. The emission spectra of various atoms. The three atomic emission spectra for oxygen can be shown on graph bellow: Strong lines of oxygen ( o ) strong lines of oxygen ( o ) intensity. 60 a diode laser was. An analyte in an excited state possesses an energy, e2, that is. Spectroscopy Emission Oxygen.
From www.researchgate.net
Emission spectra of temperatureand oxygensensing membranes as Spectroscopy Emission Oxygen The three atomic emission spectra for oxygen can be shown on graph bellow: Strong lines of oxygen ( o ) strong lines of oxygen ( o ) intensity. An analyte in an excited state possesses an energy, e2, that is greater than its energy when it is in a lower energy state, e1. When hydrogen gas is placed into a. Spectroscopy Emission Oxygen.
From www.shutterstock.com
Absorption Emission Spectrum Oxygen เวกเตอร์สต็อก (ปลอดค่าลิขสิทธิ์ Spectroscopy Emission Oxygen The three atomic emission spectra for oxygen can be shown on graph bellow: Oxygen was also sensed directly by employing gas correlation absorption spectroscopy using multimode diode lasers. The emission spectra of various atoms. Strong lines of oxygen ( o ) strong lines of oxygen ( o ) intensity. The emission spectrum (or line spectrum) of a chemical element is. Spectroscopy Emission Oxygen.
From www.researchgate.net
Absorbance and emission spectra for oxygen sensors. The absorbance and Spectroscopy Emission Oxygen Emission and absorption spectra form the basis of spectroscopy, which uses spectra to provide information about the structure and the composition of a substance or an object. 60 a diode laser was. An analyte in an excited state possesses an energy, e2, that is greater than its energy when it is in a lower energy state, e1. The emission spectra. Spectroscopy Emission Oxygen.
From brainly.com
An absorption spectrum of oxygen is shown below. What is most likely Spectroscopy Emission Oxygen When hydrogen gas is placed into a tube and. Strong lines of oxygen ( o ) strong lines of oxygen ( o ) intensity. An analyte in an excited state possesses an energy, e2, that is greater than its energy when it is in a lower energy state, e1. Emission and absorption spectra form the basis of spectroscopy, which uses. Spectroscopy Emission Oxygen.
From www.researchgate.net
The emission spectra of cold atmosphericpressure plasma jet (APPJ Spectroscopy Emission Oxygen The emission spectra of various atoms. The three atomic emission spectra for oxygen can be shown on graph bellow: Strong lines of oxygen ( o ) strong lines of oxygen ( o ) intensity. Oxygen was also sensed directly by employing gas correlation absorption spectroscopy using multimode diode lasers. 60 a diode laser was. An analyte in an excited state. Spectroscopy Emission Oxygen.
From atelier-yuwa.ciao.jp
Emission Spectra Of (A) Pure O2 Plasma Discharge And (B) Plasma Spectroscopy Emission Oxygen The three atomic emission spectra for oxygen can be shown on graph bellow: Strong lines of oxygen ( o ) strong lines of oxygen ( o ) intensity. The emission spectra of various atoms. Oxygen was also sensed directly by employing gas correlation absorption spectroscopy using multimode diode lasers. 60 a diode laser was. The emission spectrum (or line spectrum). Spectroscopy Emission Oxygen.
From www.researchgate.net
2.3. The figure shows emission spectra for oxygen (above) and argon Spectroscopy Emission Oxygen The three atomic emission spectra for oxygen can be shown on graph bellow: Emission and absorption spectra form the basis of spectroscopy, which uses spectra to provide information about the structure and the composition of a substance or an object. 60 a diode laser was. The emission spectrum (or line spectrum) of a chemical element is the unique pattern of. Spectroscopy Emission Oxygen.
From www.esa.int
ESA Absorption and emission spectra of various elements Spectroscopy Emission Oxygen Strong lines of oxygen ( o ) strong lines of oxygen ( o ) intensity. Emission and absorption spectra form the basis of spectroscopy, which uses spectra to provide information about the structure and the composition of a substance or an object. The emission spectrum (or line spectrum) of a chemical element is the unique pattern of light obtained when. Spectroscopy Emission Oxygen.
From exciting-algerien1970.blogspot.com
XRay Emission Spectra (Oxygen version) Spectroscopy Emission Oxygen The three atomic emission spectra for oxygen can be shown on graph bellow: An analyte in an excited state possesses an energy, e2, that is greater than its energy when it is in a lower energy state, e1. The emission spectra of various atoms. 60 a diode laser was. Emission and absorption spectra form the basis of spectroscopy, which uses. Spectroscopy Emission Oxygen.
From www.researchgate.net
Oxygen absorption bands and electronic transitions in the optical range Spectroscopy Emission Oxygen Emission and absorption spectra form the basis of spectroscopy, which uses spectra to provide information about the structure and the composition of a substance or an object. 60 a diode laser was. The emission spectrum (or line spectrum) of a chemical element is the unique pattern of light obtained when the element is subjected to heat or electricity. An analyte. Spectroscopy Emission Oxygen.
From www.researchgate.net
Optical emission spectroscopy (OES) spectrum of Ar/O2 plasma jet Spectroscopy Emission Oxygen 60 a diode laser was. The emission spectra of various atoms. The three atomic emission spectra for oxygen can be shown on graph bellow: When hydrogen gas is placed into a tube and. Oxygen was also sensed directly by employing gas correlation absorption spectroscopy using multimode diode lasers. Strong lines of oxygen ( o ) strong lines of oxygen (. Spectroscopy Emission Oxygen.
From www.researchgate.net
(a) Emission spectrum of oxygen at 0.95 Torr, 35 mA, 1800 V recorded Spectroscopy Emission Oxygen When hydrogen gas is placed into a tube and. The emission spectrum (or line spectrum) of a chemical element is the unique pattern of light obtained when the element is subjected to heat or electricity. An analyte in an excited state possesses an energy, e2, that is greater than its energy when it is in a lower energy state, e1.. Spectroscopy Emission Oxygen.
From wisc.pb.unizin.org
Emission Spectra and H Atom Levels (M7Q3) UWMadison Chemistry 103/ Spectroscopy Emission Oxygen The three atomic emission spectra for oxygen can be shown on graph bellow: Emission and absorption spectra form the basis of spectroscopy, which uses spectra to provide information about the structure and the composition of a substance or an object. An analyte in an excited state possesses an energy, e2, that is greater than its energy when it is in. Spectroscopy Emission Oxygen.
From innovation.ox.ac.uk
Oxygen Emission Spectroscopy Oxford University Innovation Spectroscopy Emission Oxygen When hydrogen gas is placed into a tube and. The emission spectrum (or line spectrum) of a chemical element is the unique pattern of light obtained when the element is subjected to heat or electricity. Strong lines of oxygen ( o ) strong lines of oxygen ( o ) intensity. The three atomic emission spectra for oxygen can be shown. Spectroscopy Emission Oxygen.
From www.researchgate.net
The atmospheric emission spectrum showing the carbon dioxide band at 4 Spectroscopy Emission Oxygen Strong lines of oxygen ( o ) strong lines of oxygen ( o ) intensity. The emission spectrum (or line spectrum) of a chemical element is the unique pattern of light obtained when the element is subjected to heat or electricity. Emission and absorption spectra form the basis of spectroscopy, which uses spectra to provide information about the structure and. Spectroscopy Emission Oxygen.
From www.mdpi.com
Coatings Free FullText Spectroscopic Analysis of CF4/O2 Plasma Spectroscopy Emission Oxygen 60 a diode laser was. Oxygen was also sensed directly by employing gas correlation absorption spectroscopy using multimode diode lasers. The emission spectra of various atoms. The emission spectrum (or line spectrum) of a chemical element is the unique pattern of light obtained when the element is subjected to heat or electricity. The three atomic emission spectra for oxygen can. Spectroscopy Emission Oxygen.
From hubblesite.org
Spectroscopy Reading the Rainbow HubbleSite Spectroscopy Emission Oxygen The emission spectrum (or line spectrum) of a chemical element is the unique pattern of light obtained when the element is subjected to heat or electricity. The emission spectra of various atoms. An analyte in an excited state possesses an energy, e2, that is greater than its energy when it is in a lower energy state, e1. Oxygen was also. Spectroscopy Emission Oxygen.
From www.researchgate.net
Singlet oxygen emission spectra of H2tpp, 2a and 2c in O2saturated Spectroscopy Emission Oxygen 60 a diode laser was. The emission spectra of various atoms. Strong lines of oxygen ( o ) strong lines of oxygen ( o ) intensity. The emission spectrum (or line spectrum) of a chemical element is the unique pattern of light obtained when the element is subjected to heat or electricity. The three atomic emission spectra for oxygen can. Spectroscopy Emission Oxygen.