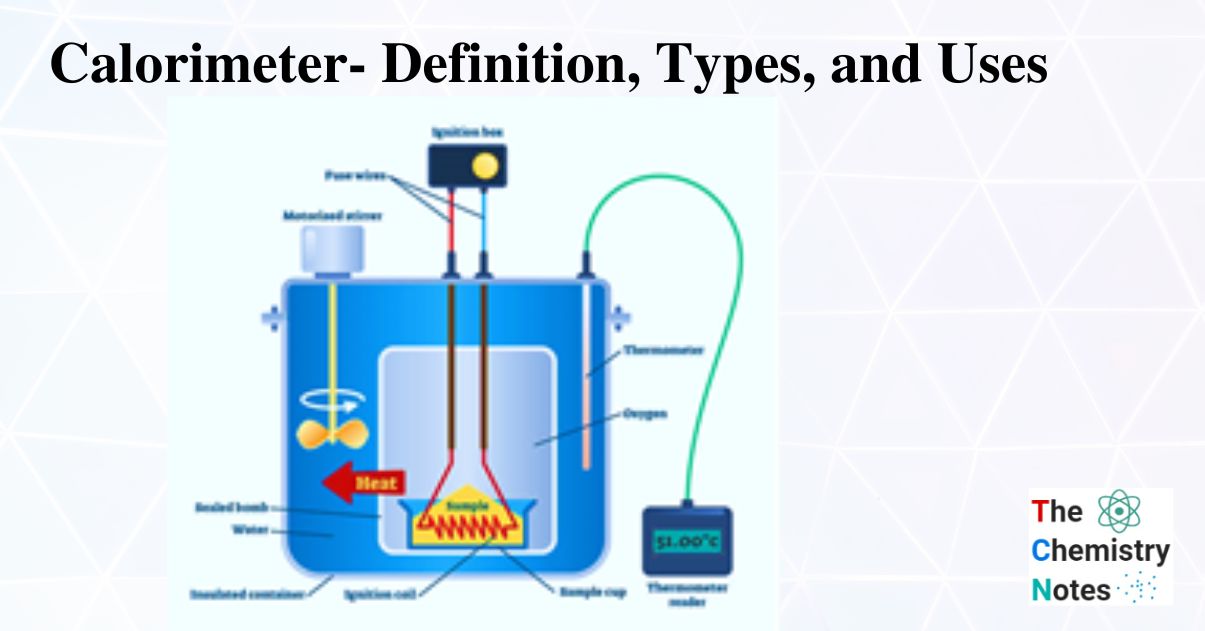
Why Calorimetry Is Important . chemists use calorimetry to determine the amount of heat transferred to or from a system into its surroundings. From a chemical reaction like. It is the only experimental method allowing for. calorimetry is a method of measuring the heat transfer within a chemical reaction or other physical processes, such as a change between. calorimetry measures enthalpy changes during chemical processes, where the magnitude of the temperature change depends on the amount of. calorimetry is the process of measuring the amount of heat released or absorbed during a chemical reaction. calorimetry belongs to the most important experimental techniques. a calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. a calorimeter is a device used to measure the amount of heat involved in a chemical or physical process.
from thechemistrynotes.com
a calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. chemists use calorimetry to determine the amount of heat transferred to or from a system into its surroundings. From a chemical reaction like. calorimetry is a method of measuring the heat transfer within a chemical reaction or other physical processes, such as a change between. It is the only experimental method allowing for. calorimetry is the process of measuring the amount of heat released or absorbed during a chemical reaction. calorimetry belongs to the most important experimental techniques. a calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. calorimetry measures enthalpy changes during chemical processes, where the magnitude of the temperature change depends on the amount of.
Calorimeter Definition, Types and Uses
Why Calorimetry Is Important From a chemical reaction like. a calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. It is the only experimental method allowing for. a calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. calorimetry is a method of measuring the heat transfer within a chemical reaction or other physical processes, such as a change between. calorimetry belongs to the most important experimental techniques. calorimetry measures enthalpy changes during chemical processes, where the magnitude of the temperature change depends on the amount of. calorimetry is the process of measuring the amount of heat released or absorbed during a chemical reaction. From a chemical reaction like. chemists use calorimetry to determine the amount of heat transferred to or from a system into its surroundings.
From quizunderfires.z21.web.core.windows.net
Why Is The Calorimeter Constant Important Why Calorimetry Is Important calorimetry belongs to the most important experimental techniques. It is the only experimental method allowing for. calorimetry is the process of measuring the amount of heat released or absorbed during a chemical reaction. calorimetry measures enthalpy changes during chemical processes, where the magnitude of the temperature change depends on the amount of. a calorimeter is a. Why Calorimetry Is Important.
From iqclasses.in
calorimetry chapter important notes class10 icse Why Calorimetry Is Important calorimetry is the process of measuring the amount of heat released or absorbed during a chemical reaction. chemists use calorimetry to determine the amount of heat transferred to or from a system into its surroundings. a calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. calorimetry is. Why Calorimetry Is Important.
From www.labster.com
5 Ways to Make Calorimetry and the Bomb Calorimeter More Approachable Why Calorimetry Is Important calorimetry is a method of measuring the heat transfer within a chemical reaction or other physical processes, such as a change between. calorimetry measures enthalpy changes during chemical processes, where the magnitude of the temperature change depends on the amount of. calorimetry belongs to the most important experimental techniques. a calorimeter is a device used to. Why Calorimetry Is Important.
From grade12uchemistry.weebly.com
Calorimetry Grade12UChemistry Why Calorimetry Is Important calorimetry measures enthalpy changes during chemical processes, where the magnitude of the temperature change depends on the amount of. a calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. calorimetry is the process of measuring the amount of heat released or absorbed during a chemical reaction. From a. Why Calorimetry Is Important.
From www.collegesearch.in
Principle of Calorimetry Definition, Formula, Principle, Types Why Calorimetry Is Important calorimetry is a method of measuring the heat transfer within a chemical reaction or other physical processes, such as a change between. chemists use calorimetry to determine the amount of heat transferred to or from a system into its surroundings. It is the only experimental method allowing for. calorimetry measures enthalpy changes during chemical processes, where the. Why Calorimetry Is Important.
From iqclasses.in
calorimetry chapter important notes class10 icse Why Calorimetry Is Important From a chemical reaction like. a calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. chemists use calorimetry to determine the amount of heat transferred to or from a system into its surroundings. calorimetry belongs to the most important experimental techniques. calorimetry is the process of measuring. Why Calorimetry Is Important.
From iqclasses.in
calorimetry chapter important notes class10 icse Why Calorimetry Is Important a calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. It is the only experimental method allowing for. calorimetry is the process of measuring the amount of heat released or absorbed during a chemical reaction. From a chemical reaction like. calorimetry belongs to the most important experimental techniques.. Why Calorimetry Is Important.
From iqclasses.in
calorimetry chapter important notes class10 icse Why Calorimetry Is Important calorimetry measures enthalpy changes during chemical processes, where the magnitude of the temperature change depends on the amount of. a calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. calorimetry is a method of measuring the heat transfer within a chemical reaction or other physical processes, such as. Why Calorimetry Is Important.
From thechemistrynotes.com
Calorimeter Definition, Types and Uses Why Calorimetry Is Important calorimetry measures enthalpy changes during chemical processes, where the magnitude of the temperature change depends on the amount of. a calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. calorimetry is the process of measuring the amount of heat released or absorbed during a chemical reaction. a. Why Calorimetry Is Important.
From www.numerade.com
SOLVED Why is it important to determine the heat capacity of the Why Calorimetry Is Important It is the only experimental method allowing for. a calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. calorimetry belongs to the most important experimental techniques. calorimetry measures enthalpy changes during chemical processes, where the magnitude of the temperature change depends on the amount of. chemists use. Why Calorimetry Is Important.
From cider.uoregon.edu
Calorimetry Heat Exchange Hot Metal in Cold Water Real and Computer Why Calorimetry Is Important calorimetry is the process of measuring the amount of heat released or absorbed during a chemical reaction. From a chemical reaction like. It is the only experimental method allowing for. a calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. chemists use calorimetry to determine the amount of. Why Calorimetry Is Important.
From www.vedantu.com
Bomb Calorimeter Learn Important Terms and Concepts Why Calorimetry Is Important calorimetry belongs to the most important experimental techniques. a calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. chemists use calorimetry to determine the amount of heat transferred to or from a system into its surroundings. calorimetry is the process of measuring the amount of heat released. Why Calorimetry Is Important.
From iqclasses.in
calorimetry chapter important notes class10 icse Why Calorimetry Is Important chemists use calorimetry to determine the amount of heat transferred to or from a system into its surroundings. a calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. calorimetry belongs to the most important experimental techniques. calorimetry is the process of measuring the amount of heat released. Why Calorimetry Is Important.
From testbook.com
Principle of Calorimetry Definition, Formula, Uses, Examples Why Calorimetry Is Important calorimetry belongs to the most important experimental techniques. calorimetry is a method of measuring the heat transfer within a chemical reaction or other physical processes, such as a change between. It is the only experimental method allowing for. a calorimeter is a device used to measure the amount of heat involved in a chemical or physical process.. Why Calorimetry Is Important.
From www.youtube.com
BASIC PRINCIPLE OF CALORIMETRY YouTube Why Calorimetry Is Important From a chemical reaction like. calorimetry measures enthalpy changes during chemical processes, where the magnitude of the temperature change depends on the amount of. calorimetry is the process of measuring the amount of heat released or absorbed during a chemical reaction. It is the only experimental method allowing for. a calorimeter is a device used to measure. Why Calorimetry Is Important.
From iqclasses.in
calorimetry chapter important notes class10 icse Why Calorimetry Is Important calorimetry is a method of measuring the heat transfer within a chemical reaction or other physical processes, such as a change between. a calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. From a chemical reaction like. a calorimeter is a device used to measure the amount of. Why Calorimetry Is Important.
From studylib.net
LAB 1 CALORIMETRY OBJECTIVES 1. Introduction to the Why Calorimetry Is Important From a chemical reaction like. chemists use calorimetry to determine the amount of heat transferred to or from a system into its surroundings. calorimetry is a method of measuring the heat transfer within a chemical reaction or other physical processes, such as a change between. calorimetry measures enthalpy changes during chemical processes, where the magnitude of the. Why Calorimetry Is Important.
From iqclasses.in
calorimetry chapter important notes class10 icse Why Calorimetry Is Important calorimetry is a method of measuring the heat transfer within a chemical reaction or other physical processes, such as a change between. It is the only experimental method allowing for. chemists use calorimetry to determine the amount of heat transferred to or from a system into its surroundings. calorimetry is the process of measuring the amount of. Why Calorimetry Is Important.
From slideplayer.com
Calorimetry. ppt download Why Calorimetry Is Important calorimetry belongs to the most important experimental techniques. a calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. calorimetry measures enthalpy changes during chemical processes, where the magnitude of the temperature change depends on the amount of. calorimetry is a method of measuring the heat transfer within. Why Calorimetry Is Important.
From www.expii.com
Bomb Calorimeter — Structure & Function Expii Why Calorimetry Is Important calorimetry is the process of measuring the amount of heat released or absorbed during a chemical reaction. calorimetry belongs to the most important experimental techniques. It is the only experimental method allowing for. a calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. From a chemical reaction like.. Why Calorimetry Is Important.
From studylib.net
Calorimetry Why Calorimetry Is Important chemists use calorimetry to determine the amount of heat transferred to or from a system into its surroundings. a calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. From a chemical reaction like. calorimetry belongs to the most important experimental techniques. It is the only experimental method allowing. Why Calorimetry Is Important.
From courses.lumenlearning.com
Calorimetry CHEM 1305 General Chemistry I—Lecture Why Calorimetry Is Important chemists use calorimetry to determine the amount of heat transferred to or from a system into its surroundings. a calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. calorimetry is a method of measuring the heat transfer within a chemical reaction or other physical processes, such as a. Why Calorimetry Is Important.
From www.tessshebaylo.com
Equation For Determining Calorimetry Tessshebaylo Why Calorimetry Is Important calorimetry measures enthalpy changes during chemical processes, where the magnitude of the temperature change depends on the amount of. From a chemical reaction like. calorimetry is a method of measuring the heat transfer within a chemical reaction or other physical processes, such as a change between. chemists use calorimetry to determine the amount of heat transferred to. Why Calorimetry Is Important.
From www.youtube.com
Thermal Properties of Matter Class 11 Physics Calorimetry Principle Why Calorimetry Is Important It is the only experimental method allowing for. a calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. From a chemical reaction like. calorimetry is the process of measuring the amount of heat released or absorbed during a chemical reaction. calorimetry measures enthalpy changes during chemical processes, where. Why Calorimetry Is Important.
From iqclasses.in
calorimetry chapter important notes class10 icse Why Calorimetry Is Important chemists use calorimetry to determine the amount of heat transferred to or from a system into its surroundings. a calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. calorimetry is a method of measuring the heat transfer within a chemical reaction or other physical processes, such as a. Why Calorimetry Is Important.
From studylib.net
Calorimetry Why Calorimetry Is Important From a chemical reaction like. calorimetry is a method of measuring the heat transfer within a chemical reaction or other physical processes, such as a change between. calorimetry measures enthalpy changes during chemical processes, where the magnitude of the temperature change depends on the amount of. a calorimeter is a device used to measure the amount of. Why Calorimetry Is Important.
From www.youtube.com
Principle of Calorimetry YouTube Why Calorimetry Is Important a calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. calorimetry belongs to the most important experimental techniques. chemists use calorimetry to determine the amount of heat transferred to or from a system into its surroundings. calorimetry measures enthalpy changes during chemical processes, where the magnitude of. Why Calorimetry Is Important.
From iqclasses.in
calorimetry chapter important notes class10 icse Why Calorimetry Is Important a calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. calorimetry measures enthalpy changes during chemical processes, where the magnitude of the temperature change depends on the amount of. calorimetry belongs to the most important experimental techniques. From a chemical reaction like. It is the only experimental method. Why Calorimetry Is Important.
From www.youtube.com
CHEMISTRY 101 Constant Pressure Calorimetry YouTube Why Calorimetry Is Important calorimetry measures enthalpy changes during chemical processes, where the magnitude of the temperature change depends on the amount of. calorimetry is a method of measuring the heat transfer within a chemical reaction or other physical processes, such as a change between. From a chemical reaction like. calorimetry is the process of measuring the amount of heat released. Why Calorimetry Is Important.
From study.com
Calorimetry Definition, Equation & Types Lesson Why Calorimetry Is Important a calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. From a chemical reaction like. calorimetry belongs to the most important experimental techniques. chemists use calorimetry to determine the amount of heat transferred to or from a system into its surroundings. a calorimeter is a device used. Why Calorimetry Is Important.
From iqclasses.in
calorimetry chapter important notes class10 icse Why Calorimetry Is Important a calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. It is the only experimental method allowing for. calorimetry is a method of measuring the heat transfer within a chemical reaction or other physical processes, such as a change between. calorimetry measures enthalpy changes during chemical processes, where. Why Calorimetry Is Important.
From sites.google.com
Calorimetry Preliminary HSC Chemistry Why Calorimetry Is Important From a chemical reaction like. chemists use calorimetry to determine the amount of heat transferred to or from a system into its surroundings. calorimetry is the process of measuring the amount of heat released or absorbed during a chemical reaction. calorimetry measures enthalpy changes during chemical processes, where the magnitude of the temperature change depends on the. Why Calorimetry Is Important.
From www.studocu.com
Experiment 6 Calorimetry Experiment 6 Calorimetry In this experiment Why Calorimetry Is Important a calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. calorimetry measures enthalpy changes during chemical processes, where the magnitude of the temperature change depends on the amount of. From a chemical reaction like. chemists use calorimetry to determine the amount of heat transferred to or from a. Why Calorimetry Is Important.
From dxowceosf.blob.core.windows.net
Calorimetry All Formulas at Spencer McSwain blog Why Calorimetry Is Important calorimetry is a method of measuring the heat transfer within a chemical reaction or other physical processes, such as a change between. It is the only experimental method allowing for. calorimetry belongs to the most important experimental techniques. From a chemical reaction like. chemists use calorimetry to determine the amount of heat transferred to or from a. Why Calorimetry Is Important.
From users.highland.edu
Calorimetry Why Calorimetry Is Important calorimetry measures enthalpy changes during chemical processes, where the magnitude of the temperature change depends on the amount of. a calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. chemists use calorimetry to determine the amount of heat transferred to or from a system into its surroundings. From. Why Calorimetry Is Important.