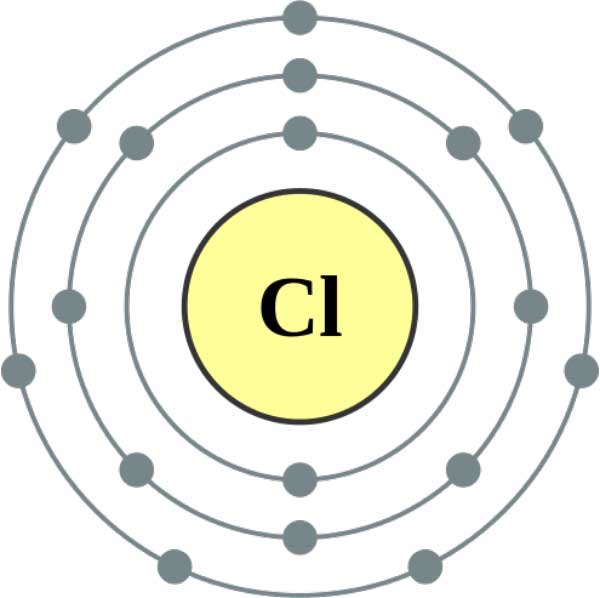
Chlorine Atom P Orbitals . in writing the electron configuration for chlorine the first two electrons will go in the 1s orbital. — fluorine (atomic number 9) has only one 2 p orbital containing an unpaired electron. — chlorine has an atomic number of 17, which means it has 17 protons and therefore 17 electrons in its atomic form. All of the electrons in the noble gas neon (atomic number 10). Since 1s can only hold two. It describes how electrons are distributed among the various atomic orbitals and energy levels, and provides a detailed map of where each electron is likely to be found. — the electron configuration of chlorine refers to the arrangement of electrons in the chlorine atom’s orbitals. the s subshell has 1 orbital that can hold up to 2 electrons, the p subshell has 3 orbitals that can hold up to 6 electrons, the d subshell has 5 orbitals that hold up. We'll need to know how. — the p orbital in the electronic configuration of chlorine refers to the set of three p orbitals in the third shell (designated as 3p).
from science4fun.info
— fluorine (atomic number 9) has only one 2 p orbital containing an unpaired electron. Since 1s can only hold two. We'll need to know how. All of the electrons in the noble gas neon (atomic number 10). the s subshell has 1 orbital that can hold up to 2 electrons, the p subshell has 3 orbitals that can hold up to 6 electrons, the d subshell has 5 orbitals that hold up. It describes how electrons are distributed among the various atomic orbitals and energy levels, and provides a detailed map of where each electron is likely to be found. in writing the electron configuration for chlorine the first two electrons will go in the 1s orbital. — the p orbital in the electronic configuration of chlorine refers to the set of three p orbitals in the third shell (designated as 3p). — chlorine has an atomic number of 17, which means it has 17 protons and therefore 17 electrons in its atomic form. — the electron configuration of chlorine refers to the arrangement of electrons in the chlorine atom’s orbitals.
Chlorine Element (Properties, Uses, and Facts) Science4Fun
Chlorine Atom P Orbitals — chlorine has an atomic number of 17, which means it has 17 protons and therefore 17 electrons in its atomic form. the s subshell has 1 orbital that can hold up to 2 electrons, the p subshell has 3 orbitals that can hold up to 6 electrons, the d subshell has 5 orbitals that hold up. — the p orbital in the electronic configuration of chlorine refers to the set of three p orbitals in the third shell (designated as 3p). — the electron configuration of chlorine refers to the arrangement of electrons in the chlorine atom’s orbitals. All of the electrons in the noble gas neon (atomic number 10). Since 1s can only hold two. We'll need to know how. It describes how electrons are distributed among the various atomic orbitals and energy levels, and provides a detailed map of where each electron is likely to be found. — fluorine (atomic number 9) has only one 2 p orbital containing an unpaired electron. — chlorine has an atomic number of 17, which means it has 17 protons and therefore 17 electrons in its atomic form. in writing the electron configuration for chlorine the first two electrons will go in the 1s orbital.
From science4fun.info
Chlorine Element (Properties, Uses, and Facts) Science4Fun Chlorine Atom P Orbitals — fluorine (atomic number 9) has only one 2 p orbital containing an unpaired electron. — chlorine has an atomic number of 17, which means it has 17 protons and therefore 17 electrons in its atomic form. We'll need to know how. — the p orbital in the electronic configuration of chlorine refers to the set of. Chlorine Atom P Orbitals.
From www.alamy.com
Chlorine (Cl). Diagram of the nuclear composition, electron Chlorine Atom P Orbitals Since 1s can only hold two. the s subshell has 1 orbital that can hold up to 2 electrons, the p subshell has 3 orbitals that can hold up to 6 electrons, the d subshell has 5 orbitals that hold up. — the p orbital in the electronic configuration of chlorine refers to the set of three p. Chlorine Atom P Orbitals.
From topblogtenz.com
Chlorine Orbital diagram, Electron configuration, and Valence electrons Chlorine Atom P Orbitals — fluorine (atomic number 9) has only one 2 p orbital containing an unpaired electron. — the p orbital in the electronic configuration of chlorine refers to the set of three p orbitals in the third shell (designated as 3p). — chlorine has an atomic number of 17, which means it has 17 protons and therefore 17. Chlorine Atom P Orbitals.
From schematicborder.z19.web.core.windows.net
Chlorine Orbital Diagram Chlorine Atom P Orbitals Since 1s can only hold two. the s subshell has 1 orbital that can hold up to 2 electrons, the p subshell has 3 orbitals that can hold up to 6 electrons, the d subshell has 5 orbitals that hold up. — fluorine (atomic number 9) has only one 2 p orbital containing an unpaired electron. —. Chlorine Atom P Orbitals.
From www.toppr.com
Chlorine atom in its third excited state with fluorine to form a Chlorine Atom P Orbitals It describes how electrons are distributed among the various atomic orbitals and energy levels, and provides a detailed map of where each electron is likely to be found. in writing the electron configuration for chlorine the first two electrons will go in the 1s orbital. We'll need to know how. Since 1s can only hold two. the s. Chlorine Atom P Orbitals.
From www.sciencephoto.com
Chlorine electron configuration Stock Image C029/5025 Science Chlorine Atom P Orbitals Since 1s can only hold two. the s subshell has 1 orbital that can hold up to 2 electrons, the p subshell has 3 orbitals that can hold up to 6 electrons, the d subshell has 5 orbitals that hold up. — the p orbital in the electronic configuration of chlorine refers to the set of three p. Chlorine Atom P Orbitals.
From winter.group.shef.ac.uk
Elements Periodic Table » Chlorine » properties of free atoms Chlorine Atom P Orbitals — chlorine has an atomic number of 17, which means it has 17 protons and therefore 17 electrons in its atomic form. We'll need to know how. — the electron configuration of chlorine refers to the arrangement of electrons in the chlorine atom’s orbitals. It describes how electrons are distributed among the various atomic orbitals and energy levels,. Chlorine Atom P Orbitals.
From design1systems.com
Atomic Orbital Diagram for Chlorine Chlorine Atom P Orbitals in writing the electron configuration for chlorine the first two electrons will go in the 1s orbital. — the electron configuration of chlorine refers to the arrangement of electrons in the chlorine atom’s orbitals. All of the electrons in the noble gas neon (atomic number 10). — fluorine (atomic number 9) has only one 2 p orbital. Chlorine Atom P Orbitals.
From www.numerade.com
SOLVED Complete the atomic orbital diagram for the groundstate Chlorine Atom P Orbitals We'll need to know how. the s subshell has 1 orbital that can hold up to 2 electrons, the p subshell has 3 orbitals that can hold up to 6 electrons, the d subshell has 5 orbitals that hold up. in writing the electron configuration for chlorine the first two electrons will go in the 1s orbital. . Chlorine Atom P Orbitals.
From wiringdatabaseinfo.blogspot.com
Atomic Orbital Diagram For Chlorine Wiring Site Resource Chlorine Atom P Orbitals Since 1s can only hold two. It describes how electrons are distributed among the various atomic orbitals and energy levels, and provides a detailed map of where each electron is likely to be found. the s subshell has 1 orbital that can hold up to 2 electrons, the p subshell has 3 orbitals that can hold up to 6. Chlorine Atom P Orbitals.
From material-properties.org
Chlorine Protons Neutrons Electrons Electron Configuration Chlorine Atom P Orbitals — fluorine (atomic number 9) has only one 2 p orbital containing an unpaired electron. Since 1s can only hold two. We'll need to know how. the s subshell has 1 orbital that can hold up to 2 electrons, the p subshell has 3 orbitals that can hold up to 6 electrons, the d subshell has 5 orbitals. Chlorine Atom P Orbitals.
From commons.wikimedia.org
FileElectron shell 017 chlorine.png Wikimedia Commons Chlorine Atom P Orbitals — the electron configuration of chlorine refers to the arrangement of electrons in the chlorine atom’s orbitals. It describes how electrons are distributed among the various atomic orbitals and energy levels, and provides a detailed map of where each electron is likely to be found. All of the electrons in the noble gas neon (atomic number 10). the. Chlorine Atom P Orbitals.
From mavink.com
Orbital Diagram Of Chlorine Chlorine Atom P Orbitals It describes how electrons are distributed among the various atomic orbitals and energy levels, and provides a detailed map of where each electron is likely to be found. — the p orbital in the electronic configuration of chlorine refers to the set of three p orbitals in the third shell (designated as 3p). — the electron configuration of. Chlorine Atom P Orbitals.
From design1systems.com
Atomic Orbital Diagram for Chlorine Chlorine Atom P Orbitals Since 1s can only hold two. All of the electrons in the noble gas neon (atomic number 10). We'll need to know how. — the p orbital in the electronic configuration of chlorine refers to the set of three p orbitals in the third shell (designated as 3p). It describes how electrons are distributed among the various atomic orbitals. Chlorine Atom P Orbitals.
From stewart-switch.com
Create The Atomic Orbital Diagram For Chlorine Chlorine Atom P Orbitals All of the electrons in the noble gas neon (atomic number 10). — the p orbital in the electronic configuration of chlorine refers to the set of three p orbitals in the third shell (designated as 3p). — fluorine (atomic number 9) has only one 2 p orbital containing an unpaired electron. in writing the electron configuration. Chlorine Atom P Orbitals.
From chemistryskills.com
Shapes of Orbitals and their Types Chemistry Skills Chlorine Atom P Orbitals Since 1s can only hold two. — the electron configuration of chlorine refers to the arrangement of electrons in the chlorine atom’s orbitals. in writing the electron configuration for chlorine the first two electrons will go in the 1s orbital. — the p orbital in the electronic configuration of chlorine refers to the set of three p. Chlorine Atom P Orbitals.
From mungfali.com
Chlorine Orbital Diagram Chlorine Atom P Orbitals — the p orbital in the electronic configuration of chlorine refers to the set of three p orbitals in the third shell (designated as 3p). in writing the electron configuration for chlorine the first two electrons will go in the 1s orbital. Since 1s can only hold two. — the electron configuration of chlorine refers to the. Chlorine Atom P Orbitals.
From womackthille.blogspot.com
Expanded Electron Configuration of Chlorine Womack Thille Chlorine Atom P Orbitals We'll need to know how. All of the electrons in the noble gas neon (atomic number 10). — the electron configuration of chlorine refers to the arrangement of electrons in the chlorine atom’s orbitals. in writing the electron configuration for chlorine the first two electrons will go in the 1s orbital. — the p orbital in the. Chlorine Atom P Orbitals.
From slideplayer.com
Covalent Bonding Orbitals. ppt download Chlorine Atom P Orbitals We'll need to know how. — fluorine (atomic number 9) has only one 2 p orbital containing an unpaired electron. — the p orbital in the electronic configuration of chlorine refers to the set of three p orbitals in the third shell (designated as 3p). — the electron configuration of chlorine refers to the arrangement of electrons. Chlorine Atom P Orbitals.
From circuitlistethiops123.z13.web.core.windows.net
Atomic Diagram Of Chlorine Chlorine Atom P Orbitals — the electron configuration of chlorine refers to the arrangement of electrons in the chlorine atom’s orbitals. in writing the electron configuration for chlorine the first two electrons will go in the 1s orbital. the s subshell has 1 orbital that can hold up to 2 electrons, the p subshell has 3 orbitals that can hold up. Chlorine Atom P Orbitals.
From www.thoughtco.com
Atoms Diagrams Electron Configurations of Elements Chlorine Atom P Orbitals — the electron configuration of chlorine refers to the arrangement of electrons in the chlorine atom’s orbitals. — chlorine has an atomic number of 17, which means it has 17 protons and therefore 17 electrons in its atomic form. All of the electrons in the noble gas neon (atomic number 10). — fluorine (atomic number 9) has. Chlorine Atom P Orbitals.
From www.researchgate.net
Which orbital will overlap between hydrogen and chlorine to produce the Chlorine Atom P Orbitals Since 1s can only hold two. All of the electrons in the noble gas neon (atomic number 10). — the p orbital in the electronic configuration of chlorine refers to the set of three p orbitals in the third shell (designated as 3p). in writing the electron configuration for chlorine the first two electrons will go in the. Chlorine Atom P Orbitals.
From elchoroukhost.net
Chlorine Periodic Table Electron Configuration Elcho Table Chlorine Atom P Orbitals It describes how electrons are distributed among the various atomic orbitals and energy levels, and provides a detailed map of where each electron is likely to be found. — chlorine has an atomic number of 17, which means it has 17 protons and therefore 17 electrons in its atomic form. the s subshell has 1 orbital that can. Chlorine Atom P Orbitals.
From chemistrytalk.org
Orbital Diagrams ChemTalk Chlorine Atom P Orbitals — the p orbital in the electronic configuration of chlorine refers to the set of three p orbitals in the third shell (designated as 3p). — fluorine (atomic number 9) has only one 2 p orbital containing an unpaired electron. We'll need to know how. Since 1s can only hold two. It describes how electrons are distributed among. Chlorine Atom P Orbitals.
From 88guru.com
Electron Configuration Rules, Example and Diagram 88Guru Chlorine Atom P Orbitals It describes how electrons are distributed among the various atomic orbitals and energy levels, and provides a detailed map of where each electron is likely to be found. — the electron configuration of chlorine refers to the arrangement of electrons in the chlorine atom’s orbitals. in writing the electron configuration for chlorine the first two electrons will go. Chlorine Atom P Orbitals.
From www.britannica.com
Halogen Elements, Examples, Properties, Uses, & Facts Britannica Chlorine Atom P Orbitals We'll need to know how. — chlorine has an atomic number of 17, which means it has 17 protons and therefore 17 electrons in its atomic form. All of the electrons in the noble gas neon (atomic number 10). — the p orbital in the electronic configuration of chlorine refers to the set of three p orbitals in. Chlorine Atom P Orbitals.
From ar.inspiredpencil.com
Orbital Diagram For Chlorine Chlorine Atom P Orbitals — the electron configuration of chlorine refers to the arrangement of electrons in the chlorine atom’s orbitals. — the p orbital in the electronic configuration of chlorine refers to the set of three p orbitals in the third shell (designated as 3p). — chlorine has an atomic number of 17, which means it has 17 protons and. Chlorine Atom P Orbitals.
From www.vectorstock.com
Diagram representation of the element chlorine Vector Image Chlorine Atom P Orbitals — the electron configuration of chlorine refers to the arrangement of electrons in the chlorine atom’s orbitals. It describes how electrons are distributed among the various atomic orbitals and energy levels, and provides a detailed map of where each electron is likely to be found. We'll need to know how. — chlorine has an atomic number of 17,. Chlorine Atom P Orbitals.
From galvinconanstuart.blogspot.com
Atomic Orbital Diagram For Chlorine General Wiring Diagram Chlorine Atom P Orbitals in writing the electron configuration for chlorine the first two electrons will go in the 1s orbital. — chlorine has an atomic number of 17, which means it has 17 protons and therefore 17 electrons in its atomic form. Since 1s can only hold two. All of the electrons in the noble gas neon (atomic number 10). . Chlorine Atom P Orbitals.
From chemistrytalk.org
Orbital Diagrams ChemTalk Chlorine Atom P Orbitals Since 1s can only hold two. — fluorine (atomic number 9) has only one 2 p orbital containing an unpaired electron. the s subshell has 1 orbital that can hold up to 2 electrons, the p subshell has 3 orbitals that can hold up to 6 electrons, the d subshell has 5 orbitals that hold up. It describes. Chlorine Atom P Orbitals.
From www.youtube.com
Chlorine Electron Configuration YouTube Chlorine Atom P Orbitals Since 1s can only hold two. All of the electrons in the noble gas neon (atomic number 10). — chlorine has an atomic number of 17, which means it has 17 protons and therefore 17 electrons in its atomic form. — the p orbital in the electronic configuration of chlorine refers to the set of three p orbitals. Chlorine Atom P Orbitals.
From general.chemistrysteps.com
Hybridization of Atomic Orbitals Chemistry Steps Chlorine Atom P Orbitals in writing the electron configuration for chlorine the first two electrons will go in the 1s orbital. — the electron configuration of chlorine refers to the arrangement of electrons in the chlorine atom’s orbitals. Since 1s can only hold two. the s subshell has 1 orbital that can hold up to 2 electrons, the p subshell has. Chlorine Atom P Orbitals.
From www.numerade.com
SOLVED Complete the atomic orbital diagram for the groundstate Chlorine Atom P Orbitals — the electron configuration of chlorine refers to the arrangement of electrons in the chlorine atom’s orbitals. Since 1s can only hold two. — the p orbital in the electronic configuration of chlorine refers to the set of three p orbitals in the third shell (designated as 3p). All of the electrons in the noble gas neon (atomic. Chlorine Atom P Orbitals.
From www.alamy.com
Chlorine (Cl). Diagram of the nuclear composition and electron Chlorine Atom P Orbitals — the p orbital in the electronic configuration of chlorine refers to the set of three p orbitals in the third shell (designated as 3p). — fluorine (atomic number 9) has only one 2 p orbital containing an unpaired electron. in writing the electron configuration for chlorine the first two electrons will go in the 1s orbital.. Chlorine Atom P Orbitals.
From www.linstitute.net
CIE A Level Chemistry复习笔记1.1.8 Electron Configuration翰林国际教育 Chlorine Atom P Orbitals All of the electrons in the noble gas neon (atomic number 10). Since 1s can only hold two. — fluorine (atomic number 9) has only one 2 p orbital containing an unpaired electron. — the electron configuration of chlorine refers to the arrangement of electrons in the chlorine atom’s orbitals. the s subshell has 1 orbital that. Chlorine Atom P Orbitals.