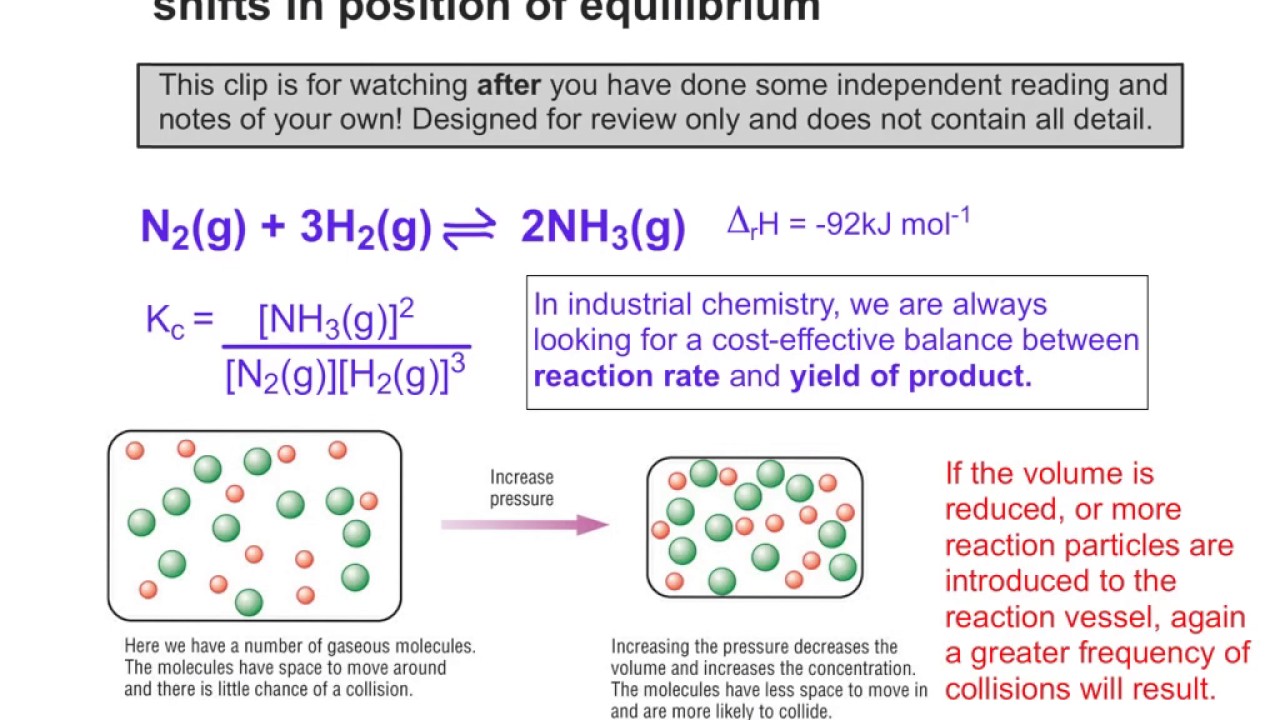
How Does Equilibrium Shift Work . Beverages are exposed to a high pressure of gaseous carbon dioxide during the process to shift the first equilibrium above to the right, resulting. Le cha telier's principle states that if a dynamic equilibrium is disturbed by changing the conditions, the position of equilibrium shifts to. An equilibrium system subjected to a disturbance. Le chatelier's principle addresses how an equilibrium shifts when the conditions of an equilibrium are changed. Addition of more product substances to an equilibrium mixture will shift the equilibrium to the left; Le chatelier’s principle addresses how an equilibrium shifts when the conditions of an equilibrium. Le chatelier's principle states that if a system in equilibrium is subjected to a change of concentration, temperature or pressure, the. This phenomenon is summarized by le châtelier’s principle: Addition of more reactant substances will. The system’s response to these disturbances is described by le châtelier’s principle:
from www.youtube.com
Beverages are exposed to a high pressure of gaseous carbon dioxide during the process to shift the first equilibrium above to the right, resulting. Le chatelier's principle states that if a system in equilibrium is subjected to a change of concentration, temperature or pressure, the. Le cha telier's principle states that if a dynamic equilibrium is disturbed by changing the conditions, the position of equilibrium shifts to. Addition of more product substances to an equilibrium mixture will shift the equilibrium to the left; Le chatelier's principle addresses how an equilibrium shifts when the conditions of an equilibrium are changed. An equilibrium system subjected to a disturbance. This phenomenon is summarized by le châtelier’s principle: Addition of more reactant substances will. Le chatelier’s principle addresses how an equilibrium shifts when the conditions of an equilibrium. The system’s response to these disturbances is described by le châtelier’s principle:
Quick review equilibrium shifts explained YouTube
How Does Equilibrium Shift Work This phenomenon is summarized by le châtelier’s principle: Le chatelier's principle addresses how an equilibrium shifts when the conditions of an equilibrium are changed. An equilibrium system subjected to a disturbance. This phenomenon is summarized by le châtelier’s principle: The system’s response to these disturbances is described by le châtelier’s principle: Addition of more reactant substances will. Le cha telier's principle states that if a dynamic equilibrium is disturbed by changing the conditions, the position of equilibrium shifts to. Le chatelier's principle states that if a system in equilibrium is subjected to a change of concentration, temperature or pressure, the. Beverages are exposed to a high pressure of gaseous carbon dioxide during the process to shift the first equilibrium above to the right, resulting. Le chatelier’s principle addresses how an equilibrium shifts when the conditions of an equilibrium. Addition of more product substances to an equilibrium mixture will shift the equilibrium to the left;
From www.youtube.com
Quick review equilibrium shifts explained YouTube How Does Equilibrium Shift Work Le chatelier's principle states that if a system in equilibrium is subjected to a change of concentration, temperature or pressure, the. This phenomenon is summarized by le châtelier’s principle: Le chatelier’s principle addresses how an equilibrium shifts when the conditions of an equilibrium. Le cha telier's principle states that if a dynamic equilibrium is disturbed by changing the conditions, the. How Does Equilibrium Shift Work.
From www.youtube.com
15 Equilibrium & Shift in equilibrium Optimization methods for How Does Equilibrium Shift Work An equilibrium system subjected to a disturbance. Addition of more product substances to an equilibrium mixture will shift the equilibrium to the left; Addition of more reactant substances will. Le chatelier's principle addresses how an equilibrium shifts when the conditions of an equilibrium are changed. Le chatelier’s principle addresses how an equilibrium shifts when the conditions of an equilibrium. This. How Does Equilibrium Shift Work.
From www.thoughtco.com
Illustrated Guide to the Supply and Demand Equilibrium How Does Equilibrium Shift Work Le chatelier's principle addresses how an equilibrium shifts when the conditions of an equilibrium are changed. This phenomenon is summarized by le châtelier’s principle: An equilibrium system subjected to a disturbance. Beverages are exposed to a high pressure of gaseous carbon dioxide during the process to shift the first equilibrium above to the right, resulting. Le cha telier's principle states. How Does Equilibrium Shift Work.
From www.slideserve.com
PPT Le Châtelier’s Principle PowerPoint Presentation, free download How Does Equilibrium Shift Work Le chatelier’s principle addresses how an equilibrium shifts when the conditions of an equilibrium. Le cha telier's principle states that if a dynamic equilibrium is disturbed by changing the conditions, the position of equilibrium shifts to. Addition of more reactant substances will. Le chatelier's principle states that if a system in equilibrium is subjected to a change of concentration, temperature. How Does Equilibrium Shift Work.
From www.slideserve.com
PPT Chemical Rxn Rates PowerPoint Presentation, free download ID How Does Equilibrium Shift Work Beverages are exposed to a high pressure of gaseous carbon dioxide during the process to shift the first equilibrium above to the right, resulting. The system’s response to these disturbances is described by le châtelier’s principle: Le cha telier's principle states that if a dynamic equilibrium is disturbed by changing the conditions, the position of equilibrium shifts to. Addition of. How Does Equilibrium Shift Work.
From www.britannica.com
Supply and demand Definition, Example, & Graph Britannica How Does Equilibrium Shift Work An equilibrium system subjected to a disturbance. The system’s response to these disturbances is described by le châtelier’s principle: Addition of more reactant substances will. Le chatelier's principle states that if a system in equilibrium is subjected to a change of concentration, temperature or pressure, the. This phenomenon is summarized by le châtelier’s principle: Le chatelier's principle addresses how an. How Does Equilibrium Shift Work.
From www.slideserve.com
PPT Chemical Equilibrium PowerPoint Presentation, free download ID How Does Equilibrium Shift Work Le chatelier's principle states that if a system in equilibrium is subjected to a change of concentration, temperature or pressure, the. Addition of more product substances to an equilibrium mixture will shift the equilibrium to the left; Addition of more reactant substances will. Beverages are exposed to a high pressure of gaseous carbon dioxide during the process to shift the. How Does Equilibrium Shift Work.
From www.chemistrystudent.com
Equilibrium (ALevel) ChemistryStudent How Does Equilibrium Shift Work Le cha telier's principle states that if a dynamic equilibrium is disturbed by changing the conditions, the position of equilibrium shifts to. The system’s response to these disturbances is described by le châtelier’s principle: Addition of more reactant substances will. Addition of more product substances to an equilibrium mixture will shift the equilibrium to the left; Le chatelier’s principle addresses. How Does Equilibrium Shift Work.
From www.youtube.com
2.08b Chemical Equilibrium How Changes Shift Equilibrium YouTube How Does Equilibrium Shift Work The system’s response to these disturbances is described by le châtelier’s principle: Addition of more product substances to an equilibrium mixture will shift the equilibrium to the left; Le chatelier's principle addresses how an equilibrium shifts when the conditions of an equilibrium are changed. Le chatelier’s principle addresses how an equilibrium shifts when the conditions of an equilibrium. Le chatelier's. How Does Equilibrium Shift Work.
From www.youtube.com
ChemC 18.2 Shifting Equilibrium YouTube How Does Equilibrium Shift Work Le cha telier's principle states that if a dynamic equilibrium is disturbed by changing the conditions, the position of equilibrium shifts to. This phenomenon is summarized by le châtelier’s principle: Addition of more product substances to an equilibrium mixture will shift the equilibrium to the left; Beverages are exposed to a high pressure of gaseous carbon dioxide during the process. How Does Equilibrium Shift Work.
From www.slideserve.com
PPT Thermochemistry PowerPoint Presentation, free download ID4251685 How Does Equilibrium Shift Work Le cha telier's principle states that if a dynamic equilibrium is disturbed by changing the conditions, the position of equilibrium shifts to. Addition of more product substances to an equilibrium mixture will shift the equilibrium to the left; Beverages are exposed to a high pressure of gaseous carbon dioxide during the process to shift the first equilibrium above to the. How Does Equilibrium Shift Work.
From sciencenotes.org
Le Chatelier's Principle How Does Equilibrium Shift Work Beverages are exposed to a high pressure of gaseous carbon dioxide during the process to shift the first equilibrium above to the right, resulting. Le chatelier's principle addresses how an equilibrium shifts when the conditions of an equilibrium are changed. Le chatelier's principle states that if a system in equilibrium is subjected to a change of concentration, temperature or pressure,. How Does Equilibrium Shift Work.
From www.slideserve.com
PPT Chemistry 142 Chapter 14 Chemical Equilibrium Review How Does Equilibrium Shift Work Le chatelier’s principle addresses how an equilibrium shifts when the conditions of an equilibrium. Le chatelier's principle addresses how an equilibrium shifts when the conditions of an equilibrium are changed. Beverages are exposed to a high pressure of gaseous carbon dioxide during the process to shift the first equilibrium above to the right, resulting. Addition of more product substances to. How Does Equilibrium Shift Work.
From www.len.com.ng
Chemical Equilibrium Dynamic Equilibrium in Chemistry How Does Equilibrium Shift Work Le chatelier's principle states that if a system in equilibrium is subjected to a change of concentration, temperature or pressure, the. This phenomenon is summarized by le châtelier’s principle: Le cha telier's principle states that if a dynamic equilibrium is disturbed by changing the conditions, the position of equilibrium shifts to. Addition of more product substances to an equilibrium mixture. How Does Equilibrium Shift Work.
From saylordotorg.github.io
Demand, Supply, and Equilibrium How Does Equilibrium Shift Work Le chatelier's principle states that if a system in equilibrium is subjected to a change of concentration, temperature or pressure, the. Addition of more product substances to an equilibrium mixture will shift the equilibrium to the left; Le chatelier’s principle addresses how an equilibrium shifts when the conditions of an equilibrium. An equilibrium system subjected to a disturbance. The system’s. How Does Equilibrium Shift Work.
From www.slideserve.com
PPT CHEMICAL EQUILIBRIUM PowerPoint Presentation, free download ID How Does Equilibrium Shift Work An equilibrium system subjected to a disturbance. The system’s response to these disturbances is described by le châtelier’s principle: Le chatelier's principle states that if a system in equilibrium is subjected to a change of concentration, temperature or pressure, the. Le chatelier’s principle addresses how an equilibrium shifts when the conditions of an equilibrium. Addition of more product substances to. How Does Equilibrium Shift Work.
From slideplayer.com
Chapter 14 Chemical Equilibrium ppt download How Does Equilibrium Shift Work The system’s response to these disturbances is described by le châtelier’s principle: Addition of more reactant substances will. This phenomenon is summarized by le châtelier’s principle: An equilibrium system subjected to a disturbance. Le cha telier's principle states that if a dynamic equilibrium is disturbed by changing the conditions, the position of equilibrium shifts to. Beverages are exposed to a. How Does Equilibrium Shift Work.
From courses.lumenlearning.com
Equilibrium, Price, and Quantity Introduction to Business How Does Equilibrium Shift Work Addition of more product substances to an equilibrium mixture will shift the equilibrium to the left; The system’s response to these disturbances is described by le châtelier’s principle: This phenomenon is summarized by le châtelier’s principle: Le chatelier's principle addresses how an equilibrium shifts when the conditions of an equilibrium are changed. Le cha telier's principle states that if a. How Does Equilibrium Shift Work.
From www.slideserve.com
PPT Le Châtelier’s Principle PowerPoint Presentation, free download How Does Equilibrium Shift Work Le cha telier's principle states that if a dynamic equilibrium is disturbed by changing the conditions, the position of equilibrium shifts to. Le chatelier's principle states that if a system in equilibrium is subjected to a change of concentration, temperature or pressure, the. Le chatelier’s principle addresses how an equilibrium shifts when the conditions of an equilibrium. Addition of more. How Does Equilibrium Shift Work.
From ilearnthis.com
What is Shift in Demand Curve? Examples & Factors How Does Equilibrium Shift Work The system’s response to these disturbances is described by le châtelier’s principle: Le chatelier’s principle addresses how an equilibrium shifts when the conditions of an equilibrium. Le chatelier's principle addresses how an equilibrium shifts when the conditions of an equilibrium are changed. Addition of more product substances to an equilibrium mixture will shift the equilibrium to the left; Beverages are. How Does Equilibrium Shift Work.
From www.slideserve.com
PPT Chapter 15 Chemical Equilibrium PowerPoint Presentation, free How Does Equilibrium Shift Work This phenomenon is summarized by le châtelier’s principle: Addition of more reactant substances will. Le chatelier’s principle addresses how an equilibrium shifts when the conditions of an equilibrium. Addition of more product substances to an equilibrium mixture will shift the equilibrium to the left; Le cha telier's principle states that if a dynamic equilibrium is disturbed by changing the conditions,. How Does Equilibrium Shift Work.
From www.pinterest.com
Pin on Chemistry How Does Equilibrium Shift Work Le cha telier's principle states that if a dynamic equilibrium is disturbed by changing the conditions, the position of equilibrium shifts to. Le chatelier's principle addresses how an equilibrium shifts when the conditions of an equilibrium are changed. Le chatelier’s principle addresses how an equilibrium shifts when the conditions of an equilibrium. Beverages are exposed to a high pressure of. How Does Equilibrium Shift Work.
From ilearnthis.com
3 Steps to Analyzing Changes in Equilibrium ilearnthis How Does Equilibrium Shift Work This phenomenon is summarized by le châtelier’s principle: Le chatelier’s principle addresses how an equilibrium shifts when the conditions of an equilibrium. Addition of more product substances to an equilibrium mixture will shift the equilibrium to the left; The system’s response to these disturbances is described by le châtelier’s principle: Le chatelier's principle states that if a system in equilibrium. How Does Equilibrium Shift Work.
From general.chemistrysteps.com
Equilibrium Constant Chemistry Steps How Does Equilibrium Shift Work Le chatelier's principle states that if a system in equilibrium is subjected to a change of concentration, temperature or pressure, the. Le chatelier's principle addresses how an equilibrium shifts when the conditions of an equilibrium are changed. The system’s response to these disturbances is described by le châtelier’s principle: Addition of more reactant substances will. This phenomenon is summarized by. How Does Equilibrium Shift Work.
From msjschemclass.blogspot.com
Ms J's Chemistry Class Le Chatlier shifting equilbrium to reduce stress How Does Equilibrium Shift Work Le cha telier's principle states that if a dynamic equilibrium is disturbed by changing the conditions, the position of equilibrium shifts to. Le chatelier’s principle addresses how an equilibrium shifts when the conditions of an equilibrium. Le chatelier's principle states that if a system in equilibrium is subjected to a change of concentration, temperature or pressure, the. This phenomenon is. How Does Equilibrium Shift Work.
From www.tutor2u.net
Changes in Market Equilibrium Price Economics tutor2u How Does Equilibrium Shift Work This phenomenon is summarized by le châtelier’s principle: Le chatelier’s principle addresses how an equilibrium shifts when the conditions of an equilibrium. Le chatelier's principle addresses how an equilibrium shifts when the conditions of an equilibrium are changed. The system’s response to these disturbances is described by le châtelier’s principle: An equilibrium system subjected to a disturbance. Addition of more. How Does Equilibrium Shift Work.
From www.slideserve.com
PPT Equilibrium PowerPoint Presentation, free download ID5729485 How Does Equilibrium Shift Work This phenomenon is summarized by le châtelier’s principle: Addition of more product substances to an equilibrium mixture will shift the equilibrium to the left; Addition of more reactant substances will. Le chatelier’s principle addresses how an equilibrium shifts when the conditions of an equilibrium. The system’s response to these disturbances is described by le châtelier’s principle: Le cha telier's principle. How Does Equilibrium Shift Work.
From spureconomics.com
Market Equilibrium, Shifts and Role of Elasticity How Does Equilibrium Shift Work Addition of more reactant substances will. This phenomenon is summarized by le châtelier’s principle: Le chatelier's principle addresses how an equilibrium shifts when the conditions of an equilibrium are changed. Addition of more product substances to an equilibrium mixture will shift the equilibrium to the left; Beverages are exposed to a high pressure of gaseous carbon dioxide during the process. How Does Equilibrium Shift Work.
From www.youtube.com
18.2 Shifting Equilibrium YouTube How Does Equilibrium Shift Work This phenomenon is summarized by le châtelier’s principle: Le chatelier's principle addresses how an equilibrium shifts when the conditions of an equilibrium are changed. Addition of more product substances to an equilibrium mixture will shift the equilibrium to the left; Le chatelier's principle states that if a system in equilibrium is subjected to a change of concentration, temperature or pressure,. How Does Equilibrium Shift Work.
From econport.gsu.edu
EconPort Shifts Shown Graphically How Does Equilibrium Shift Work Le chatelier's principle addresses how an equilibrium shifts when the conditions of an equilibrium are changed. Addition of more product substances to an equilibrium mixture will shift the equilibrium to the left; This phenomenon is summarized by le châtelier’s principle: Le chatelier’s principle addresses how an equilibrium shifts when the conditions of an equilibrium. An equilibrium system subjected to a. How Does Equilibrium Shift Work.
From www.slideserve.com
PPT Chapter 3 Market Equilibrium PowerPoint Presentation, free How Does Equilibrium Shift Work This phenomenon is summarized by le châtelier’s principle: An equilibrium system subjected to a disturbance. Beverages are exposed to a high pressure of gaseous carbon dioxide during the process to shift the first equilibrium above to the right, resulting. Le chatelier's principle addresses how an equilibrium shifts when the conditions of an equilibrium are changed. Addition of more reactant substances. How Does Equilibrium Shift Work.
From articles.outlier.org
Predicting Changes in Equilibrium Price and Quantity Outlier How Does Equilibrium Shift Work Addition of more product substances to an equilibrium mixture will shift the equilibrium to the left; Le chatelier's principle addresses how an equilibrium shifts when the conditions of an equilibrium are changed. Le chatelier’s principle addresses how an equilibrium shifts when the conditions of an equilibrium. An equilibrium system subjected to a disturbance. The system’s response to these disturbances is. How Does Equilibrium Shift Work.
From saylordotorg.github.io
Demand, Supply, and Equilibrium How Does Equilibrium Shift Work Le chatelier's principle addresses how an equilibrium shifts when the conditions of an equilibrium are changed. Beverages are exposed to a high pressure of gaseous carbon dioxide during the process to shift the first equilibrium above to the right, resulting. Le chatelier’s principle addresses how an equilibrium shifts when the conditions of an equilibrium. An equilibrium system subjected to a. How Does Equilibrium Shift Work.
From www.slideserve.com
PPT Chemical Equilibrium PowerPoint Presentation, free download ID How Does Equilibrium Shift Work Le chatelier’s principle addresses how an equilibrium shifts when the conditions of an equilibrium. Beverages are exposed to a high pressure of gaseous carbon dioxide during the process to shift the first equilibrium above to the right, resulting. This phenomenon is summarized by le châtelier’s principle: Addition of more product substances to an equilibrium mixture will shift the equilibrium to. How Does Equilibrium Shift Work.
From www.youtube.com
Reaction Quotient and Equilibrium Shift YouTube How Does Equilibrium Shift Work Le cha telier's principle states that if a dynamic equilibrium is disturbed by changing the conditions, the position of equilibrium shifts to. The system’s response to these disturbances is described by le châtelier’s principle: Addition of more product substances to an equilibrium mixture will shift the equilibrium to the left; This phenomenon is summarized by le châtelier’s principle: Le chatelier's. How Does Equilibrium Shift Work.