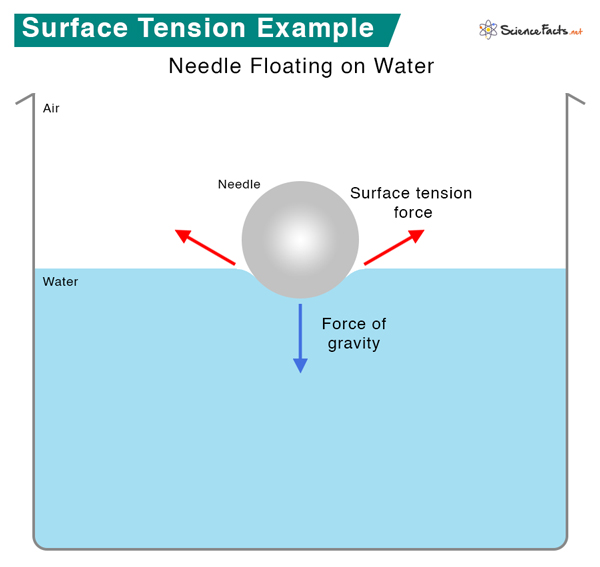
What Does A High Surface Tension Do To The Number Of Liquid Molecules . Surface tension depends mainly upon the forces of attraction between the particles within the given liquid and also upon the gas, solid, or liquid in contact with it. Among common liquids, water exhibits a distinctly high surface tension due to strong hydrogen bonding between its molecules. Surface tension is a phenomenon that occurs due to the cohesive forces of liquid molecules. The surface tension of a liquid results from an imbalance of intermolecular attractive forces, the cohesive forces between molecules: The molecules on the surface of a liquid are attracted by their neighbors from the. A molecule in the bulk liquid experiences cohesive. Surface tension is the energy required to increase the surface area of a liquid by a given amount. Surface tension is the energy, or work, required to increase the surface area of a liquid due to intermolecular forces. The stronger the intermolecular interactions, the. The cohesive forces between liquid molecules are responsible for the.
from www.sciencefacts.net
Among common liquids, water exhibits a distinctly high surface tension due to strong hydrogen bonding between its molecules. The cohesive forces between liquid molecules are responsible for the. The molecules on the surface of a liquid are attracted by their neighbors from the. Surface tension is the energy required to increase the surface area of a liquid by a given amount. A molecule in the bulk liquid experiences cohesive. Surface tension is a phenomenon that occurs due to the cohesive forces of liquid molecules. The stronger the intermolecular interactions, the. Surface tension depends mainly upon the forces of attraction between the particles within the given liquid and also upon the gas, solid, or liquid in contact with it. The surface tension of a liquid results from an imbalance of intermolecular attractive forces, the cohesive forces between molecules: Surface tension is the energy, or work, required to increase the surface area of a liquid due to intermolecular forces.
Surface Tension Definition, Examples, and Unit
What Does A High Surface Tension Do To The Number Of Liquid Molecules The cohesive forces between liquid molecules are responsible for the. Surface tension is the energy required to increase the surface area of a liquid by a given amount. The stronger the intermolecular interactions, the. A molecule in the bulk liquid experiences cohesive. Among common liquids, water exhibits a distinctly high surface tension due to strong hydrogen bonding between its molecules. Surface tension depends mainly upon the forces of attraction between the particles within the given liquid and also upon the gas, solid, or liquid in contact with it. The molecules on the surface of a liquid are attracted by their neighbors from the. Surface tension is a phenomenon that occurs due to the cohesive forces of liquid molecules. The cohesive forces between liquid molecules are responsible for the. Surface tension is the energy, or work, required to increase the surface area of a liquid due to intermolecular forces. The surface tension of a liquid results from an imbalance of intermolecular attractive forces, the cohesive forces between molecules:
From vectormine.com
Surface tension explanation vector illustration diagram VectorMine What Does A High Surface Tension Do To The Number Of Liquid Molecules Surface tension is a phenomenon that occurs due to the cohesive forces of liquid molecules. Surface tension depends mainly upon the forces of attraction between the particles within the given liquid and also upon the gas, solid, or liquid in contact with it. Among common liquids, water exhibits a distinctly high surface tension due to strong hydrogen bonding between its. What Does A High Surface Tension Do To The Number Of Liquid Molecules.
From www.slideserve.com
PPT Forces of attraction/repulsion between molecules. PowerPoint What Does A High Surface Tension Do To The Number Of Liquid Molecules Surface tension is the energy, or work, required to increase the surface area of a liquid due to intermolecular forces. The cohesive forces between liquid molecules are responsible for the. The stronger the intermolecular interactions, the. A molecule in the bulk liquid experiences cohesive. Surface tension depends mainly upon the forces of attraction between the particles within the given liquid. What Does A High Surface Tension Do To The Number Of Liquid Molecules.
From byjus.com
Explain the surface tension phenomenon with examples. What Does A High Surface Tension Do To The Number Of Liquid Molecules Surface tension is the energy required to increase the surface area of a liquid by a given amount. The molecules on the surface of a liquid are attracted by their neighbors from the. The surface tension of a liquid results from an imbalance of intermolecular attractive forces, the cohesive forces between molecules: Surface tension is a phenomenon that occurs due. What Does A High Surface Tension Do To The Number Of Liquid Molecules.
From www.slideserve.com
PPT Intermolecular Forces and Liquids and Solids PowerPoint What Does A High Surface Tension Do To The Number Of Liquid Molecules Surface tension depends mainly upon the forces of attraction between the particles within the given liquid and also upon the gas, solid, or liquid in contact with it. The cohesive forces between liquid molecules are responsible for the. The stronger the intermolecular interactions, the. Among common liquids, water exhibits a distinctly high surface tension due to strong hydrogen bonding between. What Does A High Surface Tension Do To The Number Of Liquid Molecules.
From www.sciencefacts.net
Surface Tension Definition, Examples, and Unit What Does A High Surface Tension Do To The Number Of Liquid Molecules Surface tension is a phenomenon that occurs due to the cohesive forces of liquid molecules. The cohesive forces between liquid molecules are responsible for the. A molecule in the bulk liquid experiences cohesive. Surface tension is the energy, or work, required to increase the surface area of a liquid due to intermolecular forces. Surface tension is the energy required to. What Does A High Surface Tension Do To The Number Of Liquid Molecules.
From www.slideserve.com
PPT Intermolecular Forces, Liquids and Solids PowerPoint Presentation What Does A High Surface Tension Do To The Number Of Liquid Molecules The cohesive forces between liquid molecules are responsible for the. The molecules on the surface of a liquid are attracted by their neighbors from the. Surface tension is a phenomenon that occurs due to the cohesive forces of liquid molecules. Surface tension is the energy required to increase the surface area of a liquid by a given amount. Among common. What Does A High Surface Tension Do To The Number Of Liquid Molecules.
From www.youtube.com
Surface Tension What is it, how does it form, what properties does it What Does A High Surface Tension Do To The Number Of Liquid Molecules The stronger the intermolecular interactions, the. Surface tension is the energy, or work, required to increase the surface area of a liquid due to intermolecular forces. A molecule in the bulk liquid experiences cohesive. Surface tension is the energy required to increase the surface area of a liquid by a given amount. Surface tension depends mainly upon the forces of. What Does A High Surface Tension Do To The Number Of Liquid Molecules.
From www.slideserve.com
PPT Chapter 15 PowerPoint Presentation ID245553 What Does A High Surface Tension Do To The Number Of Liquid Molecules Surface tension is the energy, or work, required to increase the surface area of a liquid due to intermolecular forces. The molecules on the surface of a liquid are attracted by their neighbors from the. Surface tension depends mainly upon the forces of attraction between the particles within the given liquid and also upon the gas, solid, or liquid in. What Does A High Surface Tension Do To The Number Of Liquid Molecules.
From bio.libretexts.org
4.5.1.3 CohesionTension Theory Biology LibreTexts What Does A High Surface Tension Do To The Number Of Liquid Molecules The surface tension of a liquid results from an imbalance of intermolecular attractive forces, the cohesive forces between molecules: The molecules on the surface of a liquid are attracted by their neighbors from the. Surface tension is the energy, or work, required to increase the surface area of a liquid due to intermolecular forces. Surface tension depends mainly upon the. What Does A High Surface Tension Do To The Number Of Liquid Molecules.
From eduinput.com
Surface TensionDefinition, Examples, Causes, And Measurement What Does A High Surface Tension Do To The Number Of Liquid Molecules Surface tension is the energy required to increase the surface area of a liquid by a given amount. Surface tension depends mainly upon the forces of attraction between the particles within the given liquid and also upon the gas, solid, or liquid in contact with it. The cohesive forces between liquid molecules are responsible for the. A molecule in the. What Does A High Surface Tension Do To The Number Of Liquid Molecules.
From www.slideserve.com
PPT LIQUID SURFACES PowerPoint Presentation, free download ID4456243 What Does A High Surface Tension Do To The Number Of Liquid Molecules Surface tension is a phenomenon that occurs due to the cohesive forces of liquid molecules. The cohesive forces between liquid molecules are responsible for the. Among common liquids, water exhibits a distinctly high surface tension due to strong hydrogen bonding between its molecules. The stronger the intermolecular interactions, the. The molecules on the surface of a liquid are attracted by. What Does A High Surface Tension Do To The Number Of Liquid Molecules.
From ideal.accelerate-ed.com
Surface Tension What Does A High Surface Tension Do To The Number Of Liquid Molecules The surface tension of a liquid results from an imbalance of intermolecular attractive forces, the cohesive forces between molecules: Surface tension is a phenomenon that occurs due to the cohesive forces of liquid molecules. Among common liquids, water exhibits a distinctly high surface tension due to strong hydrogen bonding between its molecules. The cohesive forces between liquid molecules are responsible. What Does A High Surface Tension Do To The Number Of Liquid Molecules.
From www.slideserve.com
PPT Surface Tension PowerPoint Presentation, free download ID3106425 What Does A High Surface Tension Do To The Number Of Liquid Molecules Surface tension depends mainly upon the forces of attraction between the particles within the given liquid and also upon the gas, solid, or liquid in contact with it. Surface tension is the energy, or work, required to increase the surface area of a liquid due to intermolecular forces. Among common liquids, water exhibits a distinctly high surface tension due to. What Does A High Surface Tension Do To The Number Of Liquid Molecules.
From www.vrogue.co
What Are The Importances Of Surface Tension And Visco vrogue.co What Does A High Surface Tension Do To The Number Of Liquid Molecules Surface tension is the energy required to increase the surface area of a liquid by a given amount. The stronger the intermolecular interactions, the. Surface tension is the energy, or work, required to increase the surface area of a liquid due to intermolecular forces. Surface tension depends mainly upon the forces of attraction between the particles within the given liquid. What Does A High Surface Tension Do To The Number Of Liquid Molecules.
From www.slideserve.com
PPT States of Matter PowerPoint Presentation, free download ID6036532 What Does A High Surface Tension Do To The Number Of Liquid Molecules Surface tension is the energy, or work, required to increase the surface area of a liquid due to intermolecular forces. The stronger the intermolecular interactions, the. Surface tension depends mainly upon the forces of attraction between the particles within the given liquid and also upon the gas, solid, or liquid in contact with it. Surface tension is the energy required. What Does A High Surface Tension Do To The Number Of Liquid Molecules.
From www.sliderbase.com
Properties of Liquids Presentation Chemistry What Does A High Surface Tension Do To The Number Of Liquid Molecules Among common liquids, water exhibits a distinctly high surface tension due to strong hydrogen bonding between its molecules. The molecules on the surface of a liquid are attracted by their neighbors from the. The stronger the intermolecular interactions, the. Surface tension is the energy required to increase the surface area of a liquid by a given amount. Surface tension is. What Does A High Surface Tension Do To The Number Of Liquid Molecules.
From www.slideserve.com
PPT Unit 4 The Hydrosphere PowerPoint Presentation, free download What Does A High Surface Tension Do To The Number Of Liquid Molecules Surface tension depends mainly upon the forces of attraction between the particles within the given liquid and also upon the gas, solid, or liquid in contact with it. Among common liquids, water exhibits a distinctly high surface tension due to strong hydrogen bonding between its molecules. Surface tension is the energy, or work, required to increase the surface area of. What Does A High Surface Tension Do To The Number Of Liquid Molecules.
From www.researchgate.net
a wetting state of surface with low and highsurfacetension fluids b What Does A High Surface Tension Do To The Number Of Liquid Molecules The molecules on the surface of a liquid are attracted by their neighbors from the. The surface tension of a liquid results from an imbalance of intermolecular attractive forces, the cohesive forces between molecules: The stronger the intermolecular interactions, the. Surface tension is a phenomenon that occurs due to the cohesive forces of liquid molecules. The cohesive forces between liquid. What Does A High Surface Tension Do To The Number Of Liquid Molecules.
From www.slideserve.com
PPT Physical Pharmacy SURFACE TENSION PowerPoint Presentation, free What Does A High Surface Tension Do To The Number Of Liquid Molecules Surface tension is the energy required to increase the surface area of a liquid by a given amount. Surface tension depends mainly upon the forces of attraction between the particles within the given liquid and also upon the gas, solid, or liquid in contact with it. The surface tension of a liquid results from an imbalance of intermolecular attractive forces,. What Does A High Surface Tension Do To The Number Of Liquid Molecules.
From stock.adobe.com
illustration of physics, Surface tension of water, the cohesive forces What Does A High Surface Tension Do To The Number Of Liquid Molecules Among common liquids, water exhibits a distinctly high surface tension due to strong hydrogen bonding between its molecules. Surface tension depends mainly upon the forces of attraction between the particles within the given liquid and also upon the gas, solid, or liquid in contact with it. The cohesive forces between liquid molecules are responsible for the. Surface tension is the. What Does A High Surface Tension Do To The Number Of Liquid Molecules.
From mungfali.com
Surface Tension Chart What Does A High Surface Tension Do To The Number Of Liquid Molecules The stronger the intermolecular interactions, the. The molecules on the surface of a liquid are attracted by their neighbors from the. Surface tension depends mainly upon the forces of attraction between the particles within the given liquid and also upon the gas, solid, or liquid in contact with it. Among common liquids, water exhibits a distinctly high surface tension due. What Does A High Surface Tension Do To The Number Of Liquid Molecules.
From www.slideserve.com
PPT Properties of Liquids PowerPoint Presentation, free download ID What Does A High Surface Tension Do To The Number Of Liquid Molecules Among common liquids, water exhibits a distinctly high surface tension due to strong hydrogen bonding between its molecules. Surface tension is the energy required to increase the surface area of a liquid by a given amount. The stronger the intermolecular interactions, the. The molecules on the surface of a liquid are attracted by their neighbors from the. The surface tension. What Does A High Surface Tension Do To The Number Of Liquid Molecules.
From www.geeksforgeeks.org
Surface Tension Definition, Formula, Causes, Examples, and FAQs What Does A High Surface Tension Do To The Number Of Liquid Molecules The stronger the intermolecular interactions, the. Among common liquids, water exhibits a distinctly high surface tension due to strong hydrogen bonding between its molecules. Surface tension is a phenomenon that occurs due to the cohesive forces of liquid molecules. Surface tension is the energy required to increase the surface area of a liquid by a given amount. The surface tension. What Does A High Surface Tension Do To The Number Of Liquid Molecules.
From www.biolinscientific.com
Surface tension of water Why is it so high? What Does A High Surface Tension Do To The Number Of Liquid Molecules Surface tension is the energy required to increase the surface area of a liquid by a given amount. A molecule in the bulk liquid experiences cohesive. Surface tension depends mainly upon the forces of attraction between the particles within the given liquid and also upon the gas, solid, or liquid in contact with it. Surface tension is the energy, or. What Does A High Surface Tension Do To The Number Of Liquid Molecules.
From universe-review.ca
Molecules What Does A High Surface Tension Do To The Number Of Liquid Molecules Surface tension is the energy, or work, required to increase the surface area of a liquid due to intermolecular forces. The stronger the intermolecular interactions, the. Surface tension depends mainly upon the forces of attraction between the particles within the given liquid and also upon the gas, solid, or liquid in contact with it. The cohesive forces between liquid molecules. What Does A High Surface Tension Do To The Number Of Liquid Molecules.
From www.youtube.com
Surface Tension YouTube What Does A High Surface Tension Do To The Number Of Liquid Molecules Surface tension depends mainly upon the forces of attraction between the particles within the given liquid and also upon the gas, solid, or liquid in contact with it. Among common liquids, water exhibits a distinctly high surface tension due to strong hydrogen bonding between its molecules. A molecule in the bulk liquid experiences cohesive. Surface tension is the energy, or. What Does A High Surface Tension Do To The Number Of Liquid Molecules.
From www.geeksforgeeks.org
Surface Tension Definition, Formula, Causes, Examples, and FAQs What Does A High Surface Tension Do To The Number Of Liquid Molecules Surface tension is the energy, or work, required to increase the surface area of a liquid due to intermolecular forces. The cohesive forces between liquid molecules are responsible for the. Surface tension is the energy required to increase the surface area of a liquid by a given amount. Surface tension depends mainly upon the forces of attraction between the particles. What Does A High Surface Tension Do To The Number Of Liquid Molecules.
From chem.libretexts.org
16.2 The Liquid State Chemistry LibreTexts What Does A High Surface Tension Do To The Number Of Liquid Molecules Surface tension depends mainly upon the forces of attraction between the particles within the given liquid and also upon the gas, solid, or liquid in contact with it. A molecule in the bulk liquid experiences cohesive. Surface tension is the energy required to increase the surface area of a liquid by a given amount. The surface tension of a liquid. What Does A High Surface Tension Do To The Number Of Liquid Molecules.
From www.scienceabc.com
Surface Tension Definition, Explanation, Examples And Significance What Does A High Surface Tension Do To The Number Of Liquid Molecules Among common liquids, water exhibits a distinctly high surface tension due to strong hydrogen bonding between its molecules. The cohesive forces between liquid molecules are responsible for the. The stronger the intermolecular interactions, the. Surface tension depends mainly upon the forces of attraction between the particles within the given liquid and also upon the gas, solid, or liquid in contact. What Does A High Surface Tension Do To The Number Of Liquid Molecules.
From www.slideserve.com
PPT Chapter 15 “Water and Aqueous Systems” PowerPoint Presentation What Does A High Surface Tension Do To The Number Of Liquid Molecules Among common liquids, water exhibits a distinctly high surface tension due to strong hydrogen bonding between its molecules. The stronger the intermolecular interactions, the. Surface tension is the energy required to increase the surface area of a liquid by a given amount. Surface tension depends mainly upon the forces of attraction between the particles within the given liquid and also. What Does A High Surface Tension Do To The Number Of Liquid Molecules.
From www.youtube.com
Surface Tension on the basis of Molecular theory YouTube What Does A High Surface Tension Do To The Number Of Liquid Molecules The cohesive forces between liquid molecules are responsible for the. The stronger the intermolecular interactions, the. Surface tension is the energy required to increase the surface area of a liquid by a given amount. The molecules on the surface of a liquid are attracted by their neighbors from the. Surface tension depends mainly upon the forces of attraction between the. What Does A High Surface Tension Do To The Number Of Liquid Molecules.
From readchemistry.com
Determination of Surface Tension Read Chemistry What Does A High Surface Tension Do To The Number Of Liquid Molecules The surface tension of a liquid results from an imbalance of intermolecular attractive forces, the cohesive forces between molecules: The cohesive forces between liquid molecules are responsible for the. Surface tension is a phenomenon that occurs due to the cohesive forces of liquid molecules. Among common liquids, water exhibits a distinctly high surface tension due to strong hydrogen bonding between. What Does A High Surface Tension Do To The Number Of Liquid Molecules.
From www.slideserve.com
PPT Surface Tension PowerPoint Presentation, free download ID3106425 What Does A High Surface Tension Do To The Number Of Liquid Molecules The molecules on the surface of a liquid are attracted by their neighbors from the. Surface tension depends mainly upon the forces of attraction between the particles within the given liquid and also upon the gas, solid, or liquid in contact with it. Among common liquids, water exhibits a distinctly high surface tension due to strong hydrogen bonding between its. What Does A High Surface Tension Do To The Number Of Liquid Molecules.
From www.youtube.com
Surface Tension of Water Explained YouTube What Does A High Surface Tension Do To The Number Of Liquid Molecules Surface tension depends mainly upon the forces of attraction between the particles within the given liquid and also upon the gas, solid, or liquid in contact with it. The surface tension of a liquid results from an imbalance of intermolecular attractive forces, the cohesive forces between molecules: Surface tension is the energy required to increase the surface area of a. What Does A High Surface Tension Do To The Number Of Liquid Molecules.
From www.slideserve.com
PPT Solutions PowerPoint Presentation, free download ID3227401 What Does A High Surface Tension Do To The Number Of Liquid Molecules The surface tension of a liquid results from an imbalance of intermolecular attractive forces, the cohesive forces between molecules: Among common liquids, water exhibits a distinctly high surface tension due to strong hydrogen bonding between its molecules. Surface tension is the energy required to increase the surface area of a liquid by a given amount. The molecules on the surface. What Does A High Surface Tension Do To The Number Of Liquid Molecules.