Does Distilled Water Evaporate . If the water is instead kept in a closed container, the. dissolving salts into water raises the boiling point, or in other words it increases the amount of energy required to evaporate. evaporation is the process that changes liquid water to gaseous water (water vapor). Water is a liquid because. When the surface is exposed to sunlight, some molecules gain enough energy to escape into the atmosphere. Water moves from the earth’s surface to the. evaporation is the conversion of a liquid to its vapor below the boiling temperature of the liquid. water evaporates at room temperature because the molecules at the surface of the liquid have weaker attraction than those in the bulk. the answer is yes, the rate that water evaporates can indeed be calculated, but it depends on a few more things than you. pure or distilled water evaporates faster than saltwater and other types of impure water.
from www.youtube.com
Water is a liquid because. Water moves from the earth’s surface to the. water evaporates at room temperature because the molecules at the surface of the liquid have weaker attraction than those in the bulk. the answer is yes, the rate that water evaporates can indeed be calculated, but it depends on a few more things than you. evaporation is the process that changes liquid water to gaseous water (water vapor). pure or distilled water evaporates faster than saltwater and other types of impure water. evaporation is the conversion of a liquid to its vapor below the boiling temperature of the liquid. When the surface is exposed to sunlight, some molecules gain enough energy to escape into the atmosphere. If the water is instead kept in a closed container, the. dissolving salts into water raises the boiling point, or in other words it increases the amount of energy required to evaporate.
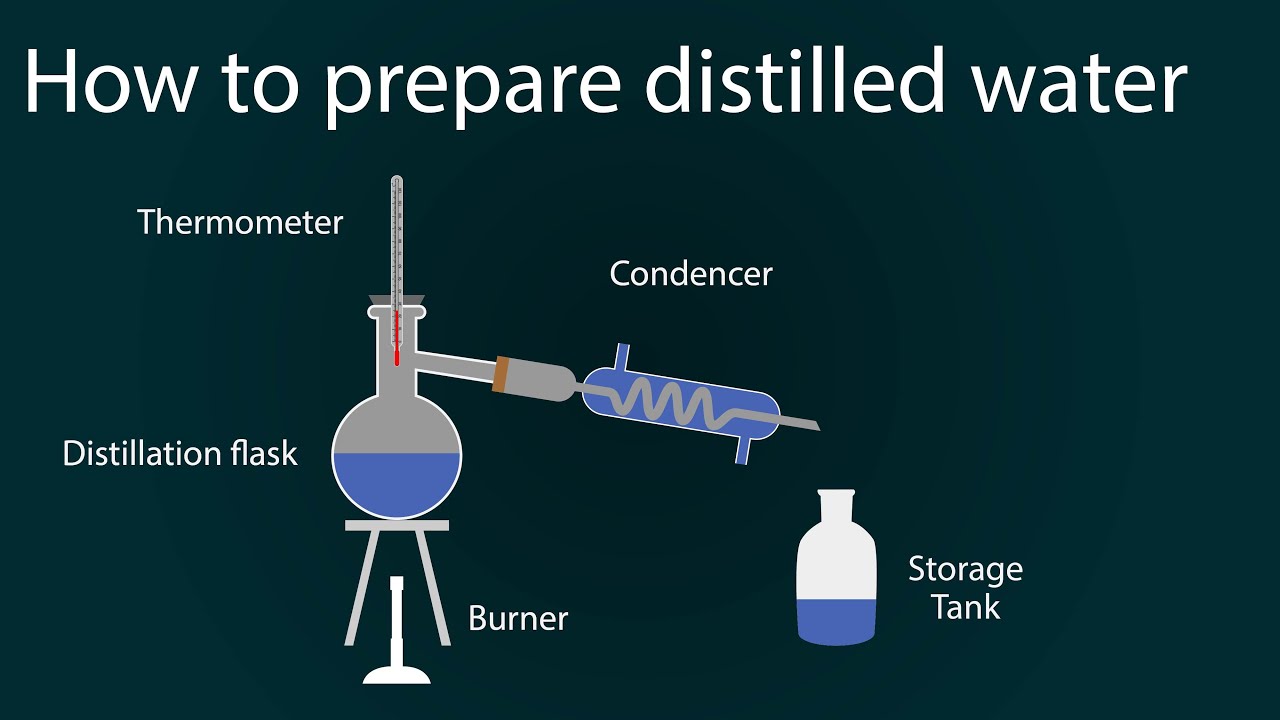
how to prepare distilled water distilled water preparation in
Does Distilled Water Evaporate pure or distilled water evaporates faster than saltwater and other types of impure water. dissolving salts into water raises the boiling point, or in other words it increases the amount of energy required to evaporate. water evaporates at room temperature because the molecules at the surface of the liquid have weaker attraction than those in the bulk. evaporation is the process that changes liquid water to gaseous water (water vapor). Water is a liquid because. When the surface is exposed to sunlight, some molecules gain enough energy to escape into the atmosphere. the answer is yes, the rate that water evaporates can indeed be calculated, but it depends on a few more things than you. If the water is instead kept in a closed container, the. pure or distilled water evaporates faster than saltwater and other types of impure water. evaporation is the conversion of a liquid to its vapor below the boiling temperature of the liquid. Water moves from the earth’s surface to the.
From www.watersmartsystems.com
Distilled Water vs Water Softener vs Filtered Water Detailed Does Distilled Water Evaporate pure or distilled water evaporates faster than saltwater and other types of impure water. Water moves from the earth’s surface to the. water evaporates at room temperature because the molecules at the surface of the liquid have weaker attraction than those in the bulk. When the surface is exposed to sunlight, some molecules gain enough energy to escape. Does Distilled Water Evaporate.
From www.alamy.com
Evaporites of hard water (left) and distilled water. Hard water Does Distilled Water Evaporate evaporation is the process that changes liquid water to gaseous water (water vapor). If the water is instead kept in a closed container, the. When the surface is exposed to sunlight, some molecules gain enough energy to escape into the atmosphere. Water is a liquid because. evaporation is the conversion of a liquid to its vapor below the. Does Distilled Water Evaporate.
From phonemantra.com
Distilled vs Purified Water Which is Right for You Does Distilled Water Evaporate dissolving salts into water raises the boiling point, or in other words it increases the amount of energy required to evaporate. If the water is instead kept in a closed container, the. evaporation is the process that changes liquid water to gaseous water (water vapor). Water is a liquid because. the answer is yes, the rate that. Does Distilled Water Evaporate.
From atonce.com
50 Unbelievable Benefits of Distilled Water Ultimate Guide 2023 Does Distilled Water Evaporate dissolving salts into water raises the boiling point, or in other words it increases the amount of energy required to evaporate. When the surface is exposed to sunlight, some molecules gain enough energy to escape into the atmosphere. the answer is yes, the rate that water evaporates can indeed be calculated, but it depends on a few more. Does Distilled Water Evaporate.
From 7esl.com
Distilled Water vs. Purified Water Differences Between these Common Does Distilled Water Evaporate evaporation is the conversion of a liquid to its vapor below the boiling temperature of the liquid. the answer is yes, the rate that water evaporates can indeed be calculated, but it depends on a few more things than you. dissolving salts into water raises the boiling point, or in other words it increases the amount of. Does Distilled Water Evaporate.
From exoetpuvj.blob.core.windows.net
Does Distilled Water Stain at Sylvia Lund blog Does Distilled Water Evaporate evaporation is the conversion of a liquid to its vapor below the boiling temperature of the liquid. evaporation is the process that changes liquid water to gaseous water (water vapor). dissolving salts into water raises the boiling point, or in other words it increases the amount of energy required to evaporate. Water moves from the earth’s surface. Does Distilled Water Evaporate.
From www.diffzy.com
Deionized vs. Distilled Water What's The Difference (With Table) Does Distilled Water Evaporate evaporation is the process that changes liquid water to gaseous water (water vapor). Water is a liquid because. pure or distilled water evaporates faster than saltwater and other types of impure water. When the surface is exposed to sunlight, some molecules gain enough energy to escape into the atmosphere. the answer is yes, the rate that water. Does Distilled Water Evaporate.
From www.verywellfit.com
Distilled Water Nutrition Facts and Health Benefits Does Distilled Water Evaporate water evaporates at room temperature because the molecules at the surface of the liquid have weaker attraction than those in the bulk. evaporation is the conversion of a liquid to its vapor below the boiling temperature of the liquid. the answer is yes, the rate that water evaporates can indeed be calculated, but it depends on a. Does Distilled Water Evaporate.
From waterseer.org
Distilled Water VS Purified Water Side Effects, Uses, And More Does Distilled Water Evaporate the answer is yes, the rate that water evaporates can indeed be calculated, but it depends on a few more things than you. When the surface is exposed to sunlight, some molecules gain enough energy to escape into the atmosphere. evaporation is the process that changes liquid water to gaseous water (water vapor). If the water is instead. Does Distilled Water Evaporate.
From pediaa.com
Difference Between Distilled Water and Purified Water Definition Does Distilled Water Evaporate When the surface is exposed to sunlight, some molecules gain enough energy to escape into the atmosphere. pure or distilled water evaporates faster than saltwater and other types of impure water. dissolving salts into water raises the boiling point, or in other words it increases the amount of energy required to evaporate. evaporation is the conversion of. Does Distilled Water Evaporate.
From www.worldatlas.com
The Water Cycle WorldAtlas Does Distilled Water Evaporate evaporation is the process that changes liquid water to gaseous water (water vapor). When the surface is exposed to sunlight, some molecules gain enough energy to escape into the atmosphere. Water is a liquid because. dissolving salts into water raises the boiling point, or in other words it increases the amount of energy required to evaporate. water. Does Distilled Water Evaporate.
From foodandkitchenappliances.com
Does Distilled Water go Bad? Everything You Need to Know Does Distilled Water Evaporate Water moves from the earth’s surface to the. water evaporates at room temperature because the molecules at the surface of the liquid have weaker attraction than those in the bulk. evaporation is the process that changes liquid water to gaseous water (water vapor). When the surface is exposed to sunlight, some molecules gain enough energy to escape into. Does Distilled Water Evaporate.
From www.chemicals.co.uk
What’s the Difference Between Distilled & Ultrapure Water? The Does Distilled Water Evaporate evaporation is the conversion of a liquid to its vapor below the boiling temperature of the liquid. the answer is yes, the rate that water evaporates can indeed be calculated, but it depends on a few more things than you. Water moves from the earth’s surface to the. If the water is instead kept in a closed container,. Does Distilled Water Evaporate.
From www.scienceabc.com
Why Does Water Evaporate At Room Temperature? Does Distilled Water Evaporate water evaporates at room temperature because the molecules at the surface of the liquid have weaker attraction than those in the bulk. Water is a liquid because. If the water is instead kept in a closed container, the. dissolving salts into water raises the boiling point, or in other words it increases the amount of energy required to. Does Distilled Water Evaporate.
From www.waterev.com
Distilled Water vs Purified Water! Which is Better for You? Does Distilled Water Evaporate If the water is instead kept in a closed container, the. When the surface is exposed to sunlight, some molecules gain enough energy to escape into the atmosphere. dissolving salts into water raises the boiling point, or in other words it increases the amount of energy required to evaporate. evaporation is the conversion of a liquid to its. Does Distilled Water Evaporate.
From www.bbc.co.uk
Evaporation BBC Bitesize Does Distilled Water Evaporate water evaporates at room temperature because the molecules at the surface of the liquid have weaker attraction than those in the bulk. pure or distilled water evaporates faster than saltwater and other types of impure water. evaporation is the conversion of a liquid to its vapor below the boiling temperature of the liquid. Water moves from the. Does Distilled Water Evaporate.
From www.livescience.com
What Is Distilled Water? Drinking Water Live Science Does Distilled Water Evaporate If the water is instead kept in a closed container, the. Water is a liquid because. When the surface is exposed to sunlight, some molecules gain enough energy to escape into the atmosphere. dissolving salts into water raises the boiling point, or in other words it increases the amount of energy required to evaporate. evaporation is the process. Does Distilled Water Evaporate.
From quenchwater.com
Distilled Water vs Purified Water Quench Water Does Distilled Water Evaporate dissolving salts into water raises the boiling point, or in other words it increases the amount of energy required to evaporate. evaporation is the conversion of a liquid to its vapor below the boiling temperature of the liquid. Water is a liquid because. the answer is yes, the rate that water evaporates can indeed be calculated, but. Does Distilled Water Evaporate.
From waterfilterguru.com
Tap Water vs Distilled Water Does Distilled Water Evaporate When the surface is exposed to sunlight, some molecules gain enough energy to escape into the atmosphere. evaporation is the conversion of a liquid to its vapor below the boiling temperature of the liquid. the answer is yes, the rate that water evaporates can indeed be calculated, but it depends on a few more things than you. . Does Distilled Water Evaporate.
From waterfilterportal.com
Distilled Water Vs Purified Water 2023 Ultimate Guide Does Distilled Water Evaporate evaporation is the process that changes liquid water to gaseous water (water vapor). dissolving salts into water raises the boiling point, or in other words it increases the amount of energy required to evaporate. water evaporates at room temperature because the molecules at the surface of the liquid have weaker attraction than those in the bulk. When. Does Distilled Water Evaporate.
From waterseer.org
Sterile Water Vs Distilled Water Know The Difference Does Distilled Water Evaporate evaporation is the conversion of a liquid to its vapor below the boiling temperature of the liquid. If the water is instead kept in a closed container, the. dissolving salts into water raises the boiling point, or in other words it increases the amount of energy required to evaporate. the answer is yes, the rate that water. Does Distilled Water Evaporate.
From microbenotes.com
Water Distiller Principle, Parts, Types, Uses, Examples Does Distilled Water Evaporate evaporation is the conversion of a liquid to its vapor below the boiling temperature of the liquid. water evaporates at room temperature because the molecules at the surface of the liquid have weaker attraction than those in the bulk. When the surface is exposed to sunlight, some molecules gain enough energy to escape into the atmosphere. Water is. Does Distilled Water Evaporate.
From waterseer.org
Distilled Water Vs Alkaline Water Know The Difference! Does Distilled Water Evaporate If the water is instead kept in a closed container, the. When the surface is exposed to sunlight, some molecules gain enough energy to escape into the atmosphere. water evaporates at room temperature because the molecules at the surface of the liquid have weaker attraction than those in the bulk. evaporation is the conversion of a liquid to. Does Distilled Water Evaporate.
From whatiswaterwebsite.com
Does Distilled Water Evaporate Faster Does Distilled Water Evaporate dissolving salts into water raises the boiling point, or in other words it increases the amount of energy required to evaporate. evaporation is the process that changes liquid water to gaseous water (water vapor). pure or distilled water evaporates faster than saltwater and other types of impure water. When the surface is exposed to sunlight, some molecules. Does Distilled Water Evaporate.
From askanydifference.com
Water vs Distilled Water Difference and Comparison Does Distilled Water Evaporate evaporation is the conversion of a liquid to its vapor below the boiling temperature of the liquid. Water moves from the earth’s surface to the. pure or distilled water evaporates faster than saltwater and other types of impure water. water evaporates at room temperature because the molecules at the surface of the liquid have weaker attraction than. Does Distilled Water Evaporate.
From www.sunrisespecialty.com
Distilled Water vs. Purified Water What's the Difference? Does Distilled Water Evaporate evaporation is the conversion of a liquid to its vapor below the boiling temperature of the liquid. evaporation is the process that changes liquid water to gaseous water (water vapor). Water moves from the earth’s surface to the. When the surface is exposed to sunlight, some molecules gain enough energy to escape into the atmosphere. pure or. Does Distilled Water Evaporate.
From nmk.world
What is Distilled Water Anyway, And How is It Different? Does Distilled Water Evaporate pure or distilled water evaporates faster than saltwater and other types of impure water. evaporation is the conversion of a liquid to its vapor below the boiling temperature of the liquid. the answer is yes, the rate that water evaporates can indeed be calculated, but it depends on a few more things than you. If the water. Does Distilled Water Evaporate.
From waterfilterguru.com
What is Distilled Water? (Everything You Need to Know) Does Distilled Water Evaporate Water is a liquid because. dissolving salts into water raises the boiling point, or in other words it increases the amount of energy required to evaporate. the answer is yes, the rate that water evaporates can indeed be calculated, but it depends on a few more things than you. pure or distilled water evaporates faster than saltwater. Does Distilled Water Evaporate.
From safewaterfilter.in
What Is Distilled Water? Does Distilled Water Evaporate Water moves from the earth’s surface to the. dissolving salts into water raises the boiling point, or in other words it increases the amount of energy required to evaporate. pure or distilled water evaporates faster than saltwater and other types of impure water. If the water is instead kept in a closed container, the. When the surface is. Does Distilled Water Evaporate.
From waterseer.org
Distilled Water Vs Deionized Water Know The Real Difference Does Distilled Water Evaporate Water moves from the earth’s surface to the. When the surface is exposed to sunlight, some molecules gain enough energy to escape into the atmosphere. If the water is instead kept in a closed container, the. evaporation is the conversion of a liquid to its vapor below the boiling temperature of the liquid. water evaporates at room temperature. Does Distilled Water Evaporate.
From waterseer.org
Nursery Water Vs Distilled Water Which is Safe For Your Baby Does Distilled Water Evaporate evaporation is the conversion of a liquid to its vapor below the boiling temperature of the liquid. dissolving salts into water raises the boiling point, or in other words it increases the amount of energy required to evaporate. Water moves from the earth’s surface to the. Water is a liquid because. When the surface is exposed to sunlight,. Does Distilled Water Evaporate.
From www.youtube.com
how to prepare distilled water distilled water preparation in Does Distilled Water Evaporate pure or distilled water evaporates faster than saltwater and other types of impure water. When the surface is exposed to sunlight, some molecules gain enough energy to escape into the atmosphere. dissolving salts into water raises the boiling point, or in other words it increases the amount of energy required to evaporate. If the water is instead kept. Does Distilled Water Evaporate.
From waterfilterspot.com
Distilled Water vs Purified Water Comparison Are They The Same? Does Distilled Water Evaporate Water is a liquid because. Water moves from the earth’s surface to the. pure or distilled water evaporates faster than saltwater and other types of impure water. dissolving salts into water raises the boiling point, or in other words it increases the amount of energy required to evaporate. When the surface is exposed to sunlight, some molecules gain. Does Distilled Water Evaporate.
From exoetpuvj.blob.core.windows.net
Does Distilled Water Stain at Sylvia Lund blog Does Distilled Water Evaporate Water is a liquid because. evaporation is the process that changes liquid water to gaseous water (water vapor). When the surface is exposed to sunlight, some molecules gain enough energy to escape into the atmosphere. dissolving salts into water raises the boiling point, or in other words it increases the amount of energy required to evaporate. If the. Does Distilled Water Evaporate.
From hadoma.com
Sterile Water Vs Distilled Water Know The Difference (2022) Does Distilled Water Evaporate If the water is instead kept in a closed container, the. pure or distilled water evaporates faster than saltwater and other types of impure water. evaporation is the conversion of a liquid to its vapor below the boiling temperature of the liquid. water evaporates at room temperature because the molecules at the surface of the liquid have. Does Distilled Water Evaporate.