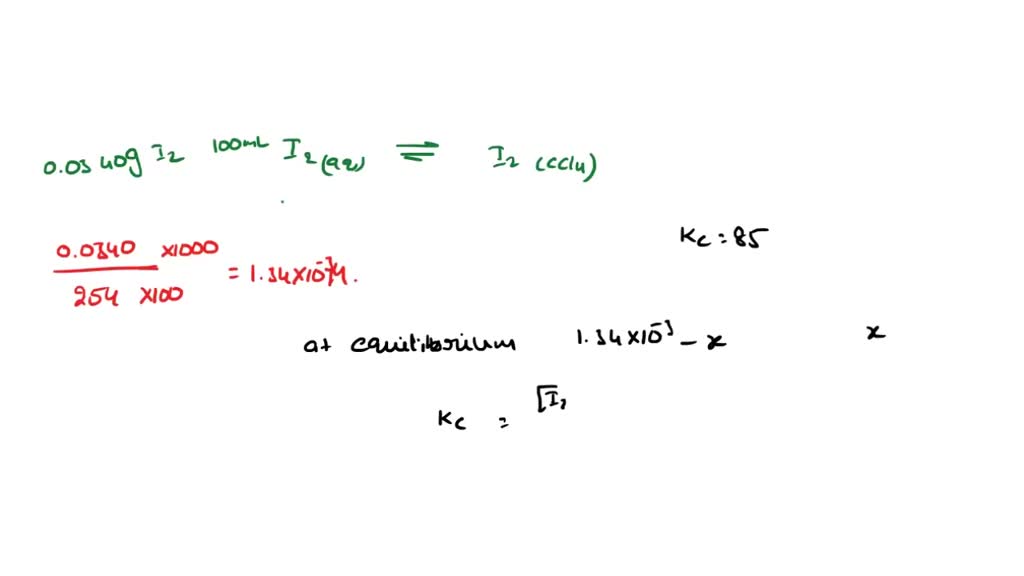What Will Dissolve In Ccl4 . So does $\ce{ccl_4}$, albeit extremely. substances that dissolve in water to yield ions are called electrolytes. Nonelectrolytes are substances that do not produce ions. The solubility of carbon tetrachloride (ccl4) in water at 25 °c is 1.2 g>l. $\ce{sicl_4}$ does not quite dissolve in water; dissolve the mixture of a and b in a solvent in which they are both soluble when hot and relatively insoluble when cold, filter off any undissolved b, and cool. The solubility of chloroform (chcl3). when a few crystals of iodine are added to the separatory funnel and the contents of the funnel are shaken, the i 2 dissolves in the ccl 4 layer to form a. Rather, it reacts with water.
from www.numerade.com
The solubility of carbon tetrachloride (ccl4) in water at 25 °c is 1.2 g>l. Nonelectrolytes are substances that do not produce ions. $\ce{sicl_4}$ does not quite dissolve in water; The solubility of chloroform (chcl3). Rather, it reacts with water. substances that dissolve in water to yield ions are called electrolytes. So does $\ce{ccl_4}$, albeit extremely. dissolve the mixture of a and b in a solvent in which they are both soluble when hot and relatively insoluble when cold, filter off any undissolved b, and cool. when a few crystals of iodine are added to the separatory funnel and the contents of the funnel are shaken, the i 2 dissolves in the ccl 4 layer to form a.
SOLVED 18. Iodine dissolves in water, but its solubility in a nonpolar
What Will Dissolve In Ccl4 Nonelectrolytes are substances that do not produce ions. Rather, it reacts with water. The solubility of carbon tetrachloride (ccl4) in water at 25 °c is 1.2 g>l. $\ce{sicl_4}$ does not quite dissolve in water; dissolve the mixture of a and b in a solvent in which they are both soluble when hot and relatively insoluble when cold, filter off any undissolved b, and cool. So does $\ce{ccl_4}$, albeit extremely. substances that dissolve in water to yield ions are called electrolytes. Nonelectrolytes are substances that do not produce ions. The solubility of chloroform (chcl3). when a few crystals of iodine are added to the separatory funnel and the contents of the funnel are shaken, the i 2 dissolves in the ccl 4 layer to form a.
From kunduz.com
[ANSWERED] why does iodine dissolve in hexane does i2 dissolve in ccl4 What Will Dissolve In Ccl4 Rather, it reacts with water. So does $\ce{ccl_4}$, albeit extremely. dissolve the mixture of a and b in a solvent in which they are both soluble when hot and relatively insoluble when cold, filter off any undissolved b, and cool. Nonelectrolytes are substances that do not produce ions. substances that dissolve in water to yield ions are called. What Will Dissolve In Ccl4.
From www.slideserve.com
PPT Chapter 7 Solutions PowerPoint Presentation, free download ID What Will Dissolve In Ccl4 The solubility of chloroform (chcl3). dissolve the mixture of a and b in a solvent in which they are both soluble when hot and relatively insoluble when cold, filter off any undissolved b, and cool. Nonelectrolytes are substances that do not produce ions. substances that dissolve in water to yield ions are called electrolytes. The solubility of carbon. What Will Dissolve In Ccl4.
From whatsinsight.org
CCl4 Lewis structure in four simple steps What's Insight What Will Dissolve In Ccl4 Nonelectrolytes are substances that do not produce ions. Rather, it reacts with water. The solubility of carbon tetrachloride (ccl4) in water at 25 °c is 1.2 g>l. dissolve the mixture of a and b in a solvent in which they are both soluble when hot and relatively insoluble when cold, filter off any undissolved b, and cool. substances. What Will Dissolve In Ccl4.
From www.numerade.com
SOLVED Question 20 Which of the following compounds would be most What Will Dissolve In Ccl4 The solubility of carbon tetrachloride (ccl4) in water at 25 °c is 1.2 g>l. Nonelectrolytes are substances that do not produce ions. Rather, it reacts with water. So does $\ce{ccl_4}$, albeit extremely. dissolve the mixture of a and b in a solvent in which they are both soluble when hot and relatively insoluble when cold, filter off any undissolved. What Will Dissolve In Ccl4.
From www.slideserve.com
PPT Packet 11 PowerPoint Presentation, free download ID6601035 What Will Dissolve In Ccl4 Nonelectrolytes are substances that do not produce ions. $\ce{sicl_4}$ does not quite dissolve in water; The solubility of chloroform (chcl3). when a few crystals of iodine are added to the separatory funnel and the contents of the funnel are shaken, the i 2 dissolves in the ccl 4 layer to form a. substances that dissolve in water. What Will Dissolve In Ccl4.
From www.slideserve.com
PPT Solutions & t heir P hysical Properties PowerPoint Presentation What Will Dissolve In Ccl4 Nonelectrolytes are substances that do not produce ions. dissolve the mixture of a and b in a solvent in which they are both soluble when hot and relatively insoluble when cold, filter off any undissolved b, and cool. when a few crystals of iodine are added to the separatory funnel and the contents of the funnel are shaken,. What Will Dissolve In Ccl4.
From www.numerade.com
SOLVED Potassium iodide reacts with iodine in aqueous solution to form What Will Dissolve In Ccl4 $\ce{sicl_4}$ does not quite dissolve in water; The solubility of carbon tetrachloride (ccl4) in water at 25 °c is 1.2 g>l. Nonelectrolytes are substances that do not produce ions. dissolve the mixture of a and b in a solvent in which they are both soluble when hot and relatively insoluble when cold, filter off any undissolved b, and. What Will Dissolve In Ccl4.
From www.chegg.com
Solved When carbon tetrachloride, CCl4 dissolves in hexane, What Will Dissolve In Ccl4 when a few crystals of iodine are added to the separatory funnel and the contents of the funnel are shaken, the i 2 dissolves in the ccl 4 layer to form a. The solubility of carbon tetrachloride (ccl4) in water at 25 °c is 1.2 g>l. $\ce{sicl_4}$ does not quite dissolve in water; Nonelectrolytes are substances that do. What Will Dissolve In Ccl4.
From www.numerade.com
SOLVED 18. Iodine dissolves in water, but its solubility in a nonpolar What Will Dissolve In Ccl4 So does $\ce{ccl_4}$, albeit extremely. dissolve the mixture of a and b in a solvent in which they are both soluble when hot and relatively insoluble when cold, filter off any undissolved b, and cool. when a few crystals of iodine are added to the separatory funnel and the contents of the funnel are shaken, the i 2. What Will Dissolve In Ccl4.
From www.numerade.com
SOLVED According to the "like dissolves like" rule, which one of the What Will Dissolve In Ccl4 Nonelectrolytes are substances that do not produce ions. $\ce{sicl_4}$ does not quite dissolve in water; So does $\ce{ccl_4}$, albeit extremely. dissolve the mixture of a and b in a solvent in which they are both soluble when hot and relatively insoluble when cold, filter off any undissolved b, and cool. The solubility of carbon tetrachloride (ccl4) in water. What Will Dissolve In Ccl4.
From www.slideserve.com
PPT Solutions PowerPoint Presentation, free download ID3154052 What Will Dissolve In Ccl4 dissolve the mixture of a and b in a solvent in which they are both soluble when hot and relatively insoluble when cold, filter off any undissolved b, and cool. So does $\ce{ccl_4}$, albeit extremely. $\ce{sicl_4}$ does not quite dissolve in water; substances that dissolve in water to yield ions are called electrolytes. The solubility of carbon. What Will Dissolve In Ccl4.
From www.numerade.com
SOLVED 1. Which of the following compounds will dissolve in CCl4? Why What Will Dissolve In Ccl4 Nonelectrolytes are substances that do not produce ions. The solubility of chloroform (chcl3). $\ce{sicl_4}$ does not quite dissolve in water; Rather, it reacts with water. dissolve the mixture of a and b in a solvent in which they are both soluble when hot and relatively insoluble when cold, filter off any undissolved b, and cool. substances that. What Will Dissolve In Ccl4.
From www.chegg.com
Solved Draw the Lewis structure for CCl4 and answer the What Will Dissolve In Ccl4 substances that dissolve in water to yield ions are called electrolytes. $\ce{sicl_4}$ does not quite dissolve in water; when a few crystals of iodine are added to the separatory funnel and the contents of the funnel are shaken, the i 2 dissolves in the ccl 4 layer to form a. Rather, it reacts with water. The solubility. What Will Dissolve In Ccl4.
From www.numerade.com
Which of the following compounds should be soluble in CCl4? a) NaCl b What Will Dissolve In Ccl4 when a few crystals of iodine are added to the separatory funnel and the contents of the funnel are shaken, the i 2 dissolves in the ccl 4 layer to form a. $\ce{sicl_4}$ does not quite dissolve in water; Nonelectrolytes are substances that do not produce ions. So does $\ce{ccl_4}$, albeit extremely. substances that dissolve in water. What Will Dissolve In Ccl4.
From www.numerade.com
SOLVED 7. (4 pts) Predict whether each of the following substances is What Will Dissolve In Ccl4 Nonelectrolytes are substances that do not produce ions. substances that dissolve in water to yield ions are called electrolytes. Rather, it reacts with water. $\ce{sicl_4}$ does not quite dissolve in water; The solubility of carbon tetrachloride (ccl4) in water at 25 °c is 1.2 g>l. dissolve the mixture of a and b in a solvent in which. What Will Dissolve In Ccl4.
From questions-in.kunduz.com
QUESTION 4 . In which of the following gas... Organic Chemistry What Will Dissolve In Ccl4 substances that dissolve in water to yield ions are called electrolytes. The solubility of chloroform (chcl3). Rather, it reacts with water. The solubility of carbon tetrachloride (ccl4) in water at 25 °c is 1.2 g>l. $\ce{sicl_4}$ does not quite dissolve in water; when a few crystals of iodine are added to the separatory funnel and the contents. What Will Dissolve In Ccl4.
From www.numerade.com
SOLVED 'The concept "like dissolves like" explains why I2 is more What Will Dissolve In Ccl4 The solubility of carbon tetrachloride (ccl4) in water at 25 °c is 1.2 g>l. So does $\ce{ccl_4}$, albeit extremely. substances that dissolve in water to yield ions are called electrolytes. dissolve the mixture of a and b in a solvent in which they are both soluble when hot and relatively insoluble when cold, filter off any undissolved b,. What Will Dissolve In Ccl4.
From slideplayer.com
Intermolecular Forces ppt download What Will Dissolve In Ccl4 when a few crystals of iodine are added to the separatory funnel and the contents of the funnel are shaken, the i 2 dissolves in the ccl 4 layer to form a. Nonelectrolytes are substances that do not produce ions. Rather, it reacts with water. The solubility of carbon tetrachloride (ccl4) in water at 25 °c is 1.2 g>l.. What Will Dissolve In Ccl4.
From www.numerade.com
SOLVED Water and carbon tetrachloride (CCl4) are liquids at room What Will Dissolve In Ccl4 The solubility of carbon tetrachloride (ccl4) in water at 25 °c is 1.2 g>l. The solubility of chloroform (chcl3). $\ce{sicl_4}$ does not quite dissolve in water; So does $\ce{ccl_4}$, albeit extremely. substances that dissolve in water to yield ions are called electrolytes. Nonelectrolytes are substances that do not produce ions. when a few crystals of iodine are. What Will Dissolve In Ccl4.
From www.numerade.com
SOLVED 18. Iodine dissolves in water, but its solubility in a nonpolar What Will Dissolve In Ccl4 dissolve the mixture of a and b in a solvent in which they are both soluble when hot and relatively insoluble when cold, filter off any undissolved b, and cool. substances that dissolve in water to yield ions are called electrolytes. Rather, it reacts with water. The solubility of carbon tetrachloride (ccl4) in water at 25 °c is. What Will Dissolve In Ccl4.
From www.numerade.com
SOLVED which of the following compounds will dissolve in CCl4? NaCl or I2 What Will Dissolve In Ccl4 Nonelectrolytes are substances that do not produce ions. dissolve the mixture of a and b in a solvent in which they are both soluble when hot and relatively insoluble when cold, filter off any undissolved b, and cool. when a few crystals of iodine are added to the separatory funnel and the contents of the funnel are shaken,. What Will Dissolve In Ccl4.
From www.studypool.com
SOLUTION Partition coefficient of iodine in water and ccl4 Studypool What Will Dissolve In Ccl4 when a few crystals of iodine are added to the separatory funnel and the contents of the funnel are shaken, the i 2 dissolves in the ccl 4 layer to form a. substances that dissolve in water to yield ions are called electrolytes. Nonelectrolytes are substances that do not produce ions. $\ce{sicl_4}$ does not quite dissolve in. What Will Dissolve In Ccl4.
From www.slideserve.com
PPT Chapter 7 Solutions PowerPoint Presentation, free download ID What Will Dissolve In Ccl4 The solubility of carbon tetrachloride (ccl4) in water at 25 °c is 1.2 g>l. $\ce{sicl_4}$ does not quite dissolve in water; dissolve the mixture of a and b in a solvent in which they are both soluble when hot and relatively insoluble when cold, filter off any undissolved b, and cool. Rather, it reacts with water. Nonelectrolytes are. What Will Dissolve In Ccl4.
From www.slideshare.net
Chem Lab 2 What Will Dissolve In Ccl4 substances that dissolve in water to yield ions are called electrolytes. when a few crystals of iodine are added to the separatory funnel and the contents of the funnel are shaken, the i 2 dissolves in the ccl 4 layer to form a. dissolve the mixture of a and b in a solvent in which they are. What Will Dissolve In Ccl4.
From www.numerade.com
SOLVED Part A The solubility of carbon tetrachloride (CCl4) in water What Will Dissolve In Ccl4 So does $\ce{ccl_4}$, albeit extremely. Rather, it reacts with water. substances that dissolve in water to yield ions are called electrolytes. The solubility of carbon tetrachloride (ccl4) in water at 25 °c is 1.2 g>l. $\ce{sicl_4}$ does not quite dissolve in water; Nonelectrolytes are substances that do not produce ions. The solubility of chloroform (chcl3). when a. What Will Dissolve In Ccl4.
From www.toppr.com
Three compounds A, B, and C are isomers of the formula C5H8 . All of What Will Dissolve In Ccl4 dissolve the mixture of a and b in a solvent in which they are both soluble when hot and relatively insoluble when cold, filter off any undissolved b, and cool. Nonelectrolytes are substances that do not produce ions. $\ce{sicl_4}$ does not quite dissolve in water; substances that dissolve in water to yield ions are called electrolytes. . What Will Dissolve In Ccl4.
From www.youtube.com
CCl4 Lewis Structure How to Draw the Dot Structure for CCl4 (Carbon What Will Dissolve In Ccl4 dissolve the mixture of a and b in a solvent in which they are both soluble when hot and relatively insoluble when cold, filter off any undissolved b, and cool. substances that dissolve in water to yield ions are called electrolytes. Rather, it reacts with water. Nonelectrolytes are substances that do not produce ions. The solubility of carbon. What Will Dissolve In Ccl4.
From www.slideserve.com
PPT Chemistry PowerPoint Presentation, free download ID650195 What Will Dissolve In Ccl4 So does $\ce{ccl_4}$, albeit extremely. The solubility of carbon tetrachloride (ccl4) in water at 25 °c is 1.2 g>l. The solubility of chloroform (chcl3). substances that dissolve in water to yield ions are called electrolytes. when a few crystals of iodine are added to the separatory funnel and the contents of the funnel are shaken, the i 2. What Will Dissolve In Ccl4.
From www.chegg.com
Solved Which of the following compounds would dissolve in What Will Dissolve In Ccl4 Rather, it reacts with water. The solubility of carbon tetrachloride (ccl4) in water at 25 °c is 1.2 g>l. when a few crystals of iodine are added to the separatory funnel and the contents of the funnel are shaken, the i 2 dissolves in the ccl 4 layer to form a. $\ce{sicl_4}$ does not quite dissolve in water;. What Will Dissolve In Ccl4.
From www.numerade.com
SOLVEDDescribe at the molecular level what is happening when solid What Will Dissolve In Ccl4 substances that dissolve in water to yield ions are called electrolytes. The solubility of carbon tetrachloride (ccl4) in water at 25 °c is 1.2 g>l. The solubility of chloroform (chcl3). when a few crystals of iodine are added to the separatory funnel and the contents of the funnel are shaken, the i 2 dissolves in the ccl 4. What Will Dissolve In Ccl4.
From www.numerade.com
SOLVED CHEMWORK Indicate whether water (H2O) or carbon tetrachloride What Will Dissolve In Ccl4 substances that dissolve in water to yield ions are called electrolytes. Nonelectrolytes are substances that do not produce ions. The solubility of chloroform (chcl3). $\ce{sicl_4}$ does not quite dissolve in water; dissolve the mixture of a and b in a solvent in which they are both soluble when hot and relatively insoluble when cold, filter off any. What Will Dissolve In Ccl4.
From www.chegg.com
Solved Question 8 Carbon tetrachloride, CCl4, is a nonpolar What Will Dissolve In Ccl4 The solubility of chloroform (chcl3). So does $\ce{ccl_4}$, albeit extremely. Nonelectrolytes are substances that do not produce ions. The solubility of carbon tetrachloride (ccl4) in water at 25 °c is 1.2 g>l. dissolve the mixture of a and b in a solvent in which they are both soluble when hot and relatively insoluble when cold, filter off any undissolved. What Will Dissolve In Ccl4.
From www.numerade.com
SOLVEDIodine dissolves in water, but its solubility in a nonpolar What Will Dissolve In Ccl4 Rather, it reacts with water. when a few crystals of iodine are added to the separatory funnel and the contents of the funnel are shaken, the i 2 dissolves in the ccl 4 layer to form a. Nonelectrolytes are substances that do not produce ions. The solubility of carbon tetrachloride (ccl4) in water at 25 °c is 1.2 g>l.. What Will Dissolve In Ccl4.
From www.slideserve.com
PPT Chapter 7 Solutions PowerPoint Presentation, free download ID What Will Dissolve In Ccl4 when a few crystals of iodine are added to the separatory funnel and the contents of the funnel are shaken, the i 2 dissolves in the ccl 4 layer to form a. $\ce{sicl_4}$ does not quite dissolve in water; Nonelectrolytes are substances that do not produce ions. dissolve the mixture of a and b in a solvent. What Will Dissolve In Ccl4.
From www.numerade.com
SOLVED Texts Determine whether the following molecules will dissolve What Will Dissolve In Ccl4 So does $\ce{ccl_4}$, albeit extremely. when a few crystals of iodine are added to the separatory funnel and the contents of the funnel are shaken, the i 2 dissolves in the ccl 4 layer to form a. The solubility of carbon tetrachloride (ccl4) in water at 25 °c is 1.2 g>l. Rather, it reacts with water. dissolve the. What Will Dissolve In Ccl4.