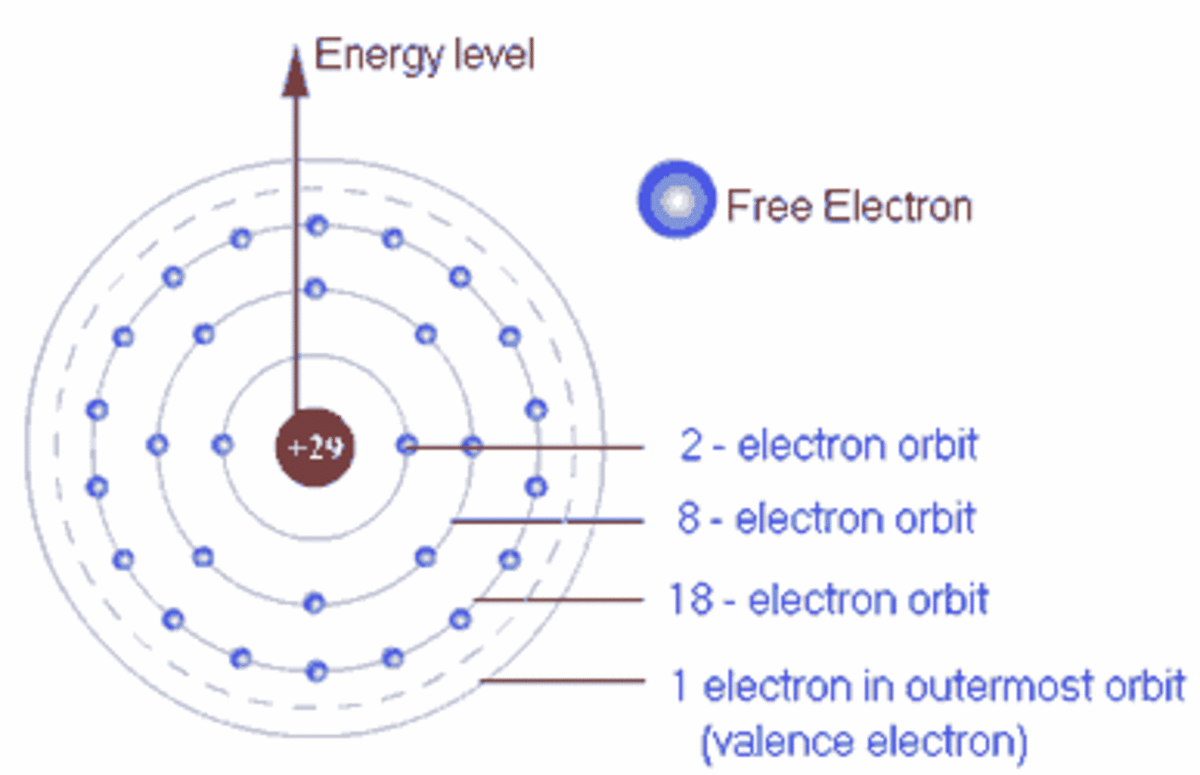
Does Iron Gain Electrons . in writing the electron configuration for iron the first two electrons will go in the 1s orbital. the initial two electrons in the electron configuration for iron will be in the 1s orbital. Because the 1s orbital can only hold two electrons, the next two. Since 1s can only hold two. When an atom loses an electron it gains a positive charge and is called a. in simple terms, the number of electrons an element can gain, lose or share to achieve a fully filled outermost electron. Some atoms have nearly eight electrons in their valence shell and can gain additional valence electrons until they. For elements in groups 6 and 7, the. the ions have the electronic structure of a noble gas (group 0 element), with a full outer shell. atoms can gain or lose electrons to become ions.
from profliway.hubpages.com
atoms can gain or lose electrons to become ions. Since 1s can only hold two. For elements in groups 6 and 7, the. Some atoms have nearly eight electrons in their valence shell and can gain additional valence electrons until they. in writing the electron configuration for iron the first two electrons will go in the 1s orbital. Because the 1s orbital can only hold two electrons, the next two. in simple terms, the number of electrons an element can gain, lose or share to achieve a fully filled outermost electron. the ions have the electronic structure of a noble gas (group 0 element), with a full outer shell. When an atom loses an electron it gains a positive charge and is called a. the initial two electrons in the electron configuration for iron will be in the 1s orbital.
Chemical Bonding How Do Atoms Combine? What Are the Forces That Bind
Does Iron Gain Electrons Some atoms have nearly eight electrons in their valence shell and can gain additional valence electrons until they. When an atom loses an electron it gains a positive charge and is called a. Since 1s can only hold two. Because the 1s orbital can only hold two electrons, the next two. in writing the electron configuration for iron the first two electrons will go in the 1s orbital. the ions have the electronic structure of a noble gas (group 0 element), with a full outer shell. Some atoms have nearly eight electrons in their valence shell and can gain additional valence electrons until they. For elements in groups 6 and 7, the. atoms can gain or lose electrons to become ions. the initial two electrons in the electron configuration for iron will be in the 1s orbital. in simple terms, the number of electrons an element can gain, lose or share to achieve a fully filled outermost electron.
From valenceelectrons.com
How to Find the Valence Electrons for Iron (Fe)? Does Iron Gain Electrons Because the 1s orbital can only hold two electrons, the next two. the ions have the electronic structure of a noble gas (group 0 element), with a full outer shell. When an atom loses an electron it gains a positive charge and is called a. the initial two electrons in the electron configuration for iron will be in. Does Iron Gain Electrons.
From sarai-kobrien.blogspot.com
Metals Tend to Lose Electrons to Positive Ions Does Iron Gain Electrons When an atom loses an electron it gains a positive charge and is called a. the initial two electrons in the electron configuration for iron will be in the 1s orbital. the ions have the electronic structure of a noble gas (group 0 element), with a full outer shell. in simple terms, the number of electrons an. Does Iron Gain Electrons.
From slideplayer.com
Chapter 4 Electron Arrangement. ppt download Does Iron Gain Electrons in writing the electron configuration for iron the first two electrons will go in the 1s orbital. When an atom loses an electron it gains a positive charge and is called a. atoms can gain or lose electrons to become ions. the initial two electrons in the electron configuration for iron will be in the 1s orbital.. Does Iron Gain Electrons.
From jacksofscience.com
Iron Valence Electrons Jacks Of Science Does Iron Gain Electrons Some atoms have nearly eight electrons in their valence shell and can gain additional valence electrons until they. in writing the electron configuration for iron the first two electrons will go in the 1s orbital. in simple terms, the number of electrons an element can gain, lose or share to achieve a fully filled outermost electron. Since 1s. Does Iron Gain Electrons.
From profliway.hubpages.com
Chemical Bonding How Do Atoms Combine? What Are the Forces That Bind Does Iron Gain Electrons Some atoms have nearly eight electrons in their valence shell and can gain additional valence electrons until they. in simple terms, the number of electrons an element can gain, lose or share to achieve a fully filled outermost electron. Since 1s can only hold two. in writing the electron configuration for iron the first two electrons will go. Does Iron Gain Electrons.
From ar.inspiredpencil.com
Iron Orbital Notation Does Iron Gain Electrons When an atom loses an electron it gains a positive charge and is called a. atoms can gain or lose electrons to become ions. Because the 1s orbital can only hold two electrons, the next two. Since 1s can only hold two. the ions have the electronic structure of a noble gas (group 0 element), with a full. Does Iron Gain Electrons.
From slideplayer.com
ppt download Does Iron Gain Electrons in writing the electron configuration for iron the first two electrons will go in the 1s orbital. Since 1s can only hold two. For elements in groups 6 and 7, the. the initial two electrons in the electron configuration for iron will be in the 1s orbital. When an atom loses an electron it gains a positive charge. Does Iron Gain Electrons.
From www.bigstockphoto.com
Iron. Atom Structure Vector & Photo (Free Trial) Bigstock Does Iron Gain Electrons For elements in groups 6 and 7, the. in simple terms, the number of electrons an element can gain, lose or share to achieve a fully filled outermost electron. in writing the electron configuration for iron the first two electrons will go in the 1s orbital. atoms can gain or lose electrons to become ions. Some atoms. Does Iron Gain Electrons.
From www.slideserve.com
PPT Oxidation an atom loses one or more electrons . PowerPoint Does Iron Gain Electrons For elements in groups 6 and 7, the. in writing the electron configuration for iron the first two electrons will go in the 1s orbital. the initial two electrons in the electron configuration for iron will be in the 1s orbital. Since 1s can only hold two. Because the 1s orbital can only hold two electrons, the next. Does Iron Gain Electrons.
From www.slideserve.com
PPT CHEMICAL FORMULAS AND BONDING PowerPoint Presentation, free Does Iron Gain Electrons Since 1s can only hold two. in writing the electron configuration for iron the first two electrons will go in the 1s orbital. the initial two electrons in the electron configuration for iron will be in the 1s orbital. Some atoms have nearly eight electrons in their valence shell and can gain additional valence electrons until they. Because. Does Iron Gain Electrons.
From www.slideserve.com
PPT Bonding Between Atoms PowerPoint Presentation, free download ID Does Iron Gain Electrons atoms can gain or lose electrons to become ions. Some atoms have nearly eight electrons in their valence shell and can gain additional valence electrons until they. in writing the electron configuration for iron the first two electrons will go in the 1s orbital. the initial two electrons in the electron configuration for iron will be in. Does Iron Gain Electrons.
From valenceelectrons.com
How Many Protons, Neutrons and Electrons Does Iron Have? Does Iron Gain Electrons in simple terms, the number of electrons an element can gain, lose or share to achieve a fully filled outermost electron. For elements in groups 6 and 7, the. atoms can gain or lose electrons to become ions. in writing the electron configuration for iron the first two electrons will go in the 1s orbital. Because the. Does Iron Gain Electrons.
From material-properties.org
Iron Protons Neutrons Electrons Electron Configuration Does Iron Gain Electrons When an atom loses an electron it gains a positive charge and is called a. Some atoms have nearly eight electrons in their valence shell and can gain additional valence electrons until they. For elements in groups 6 and 7, the. atoms can gain or lose electrons to become ions. in simple terms, the number of electrons an. Does Iron Gain Electrons.
From www.nuclear-power.com
Iron Electron Affinity Electronegativity Ionization Energy of Does Iron Gain Electrons the initial two electrons in the electron configuration for iron will be in the 1s orbital. in writing the electron configuration for iron the first two electrons will go in the 1s orbital. Some atoms have nearly eight electrons in their valence shell and can gain additional valence electrons until they. For elements in groups 6 and 7,. Does Iron Gain Electrons.
From www.youtube.com
How many valence electrons does iron have? YouTube Does Iron Gain Electrons Some atoms have nearly eight electrons in their valence shell and can gain additional valence electrons until they. Because the 1s orbital can only hold two electrons, the next two. Since 1s can only hold two. in writing the electron configuration for iron the first two electrons will go in the 1s orbital. the initial two electrons in. Does Iron Gain Electrons.
From www.dreamstime.com
Iron Atom Stock Illustrations 1,568 Iron Atom Stock Illustrations Does Iron Gain Electrons in writing the electron configuration for iron the first two electrons will go in the 1s orbital. For elements in groups 6 and 7, the. Because the 1s orbital can only hold two electrons, the next two. in simple terms, the number of electrons an element can gain, lose or share to achieve a fully filled outermost electron.. Does Iron Gain Electrons.
From slideplayer.com
Dmitri Mendeleev The Periodic Table of the Elements. ppt download Does Iron Gain Electrons the ions have the electronic structure of a noble gas (group 0 element), with a full outer shell. For elements in groups 6 and 7, the. the initial two electrons in the electron configuration for iron will be in the 1s orbital. atoms can gain or lose electrons to become ions. in simple terms, the number. Does Iron Gain Electrons.
From www.slideserve.com
PPT UNIT 1 Structure and Function (Biochemistry) Chapter 2 Does Iron Gain Electrons in simple terms, the number of electrons an element can gain, lose or share to achieve a fully filled outermost electron. the ions have the electronic structure of a noble gas (group 0 element), with a full outer shell. the initial two electrons in the electron configuration for iron will be in the 1s orbital. atoms. Does Iron Gain Electrons.
From exovwpszu.blob.core.windows.net
Iron Number Of Electrons Lost Or Gained at Anneliese Findlay blog Does Iron Gain Electrons When an atom loses an electron it gains a positive charge and is called a. Because the 1s orbital can only hold two electrons, the next two. the initial two electrons in the electron configuration for iron will be in the 1s orbital. atoms can gain or lose electrons to become ions. the ions have the electronic. Does Iron Gain Electrons.
From valenceelectrons.com
How Many Valence Electrons Does Iron (Fe) Have? Does Iron Gain Electrons the ions have the electronic structure of a noble gas (group 0 element), with a full outer shell. atoms can gain or lose electrons to become ions. the initial two electrons in the electron configuration for iron will be in the 1s orbital. in writing the electron configuration for iron the first two electrons will go. Does Iron Gain Electrons.
From www.alamy.com
Symbol and electron diagram for Iron illustration Stock Vector Image Does Iron Gain Electrons Some atoms have nearly eight electrons in their valence shell and can gain additional valence electrons until they. the initial two electrons in the electron configuration for iron will be in the 1s orbital. Since 1s can only hold two. in simple terms, the number of electrons an element can gain, lose or share to achieve a fully. Does Iron Gain Electrons.
From www.slideserve.com
PPT Chemistry 120 PowerPoint Presentation, free download ID6788469 Does Iron Gain Electrons Some atoms have nearly eight electrons in their valence shell and can gain additional valence electrons until they. in simple terms, the number of electrons an element can gain, lose or share to achieve a fully filled outermost electron. the ions have the electronic structure of a noble gas (group 0 element), with a full outer shell. . Does Iron Gain Electrons.
From www.askiitians.com
Classification of Elements & Periodicity in Properties askIITians Does Iron Gain Electrons When an atom loses an electron it gains a positive charge and is called a. Since 1s can only hold two. atoms can gain or lose electrons to become ions. Some atoms have nearly eight electrons in their valence shell and can gain additional valence electrons until they. the ions have the electronic structure of a noble gas. Does Iron Gain Electrons.
From valenceelectrons.com
Electron Configuration for Iron (Fe and Fe2+, Fe3+ ions) Does Iron Gain Electrons When an atom loses an electron it gains a positive charge and is called a. For elements in groups 6 and 7, the. Because the 1s orbital can only hold two electrons, the next two. the initial two electrons in the electron configuration for iron will be in the 1s orbital. in simple terms, the number of electrons. Does Iron Gain Electrons.
From brainly.in
which elements are likely to gain electrons ? Brainly.in Does Iron Gain Electrons atoms can gain or lose electrons to become ions. Since 1s can only hold two. Some atoms have nearly eight electrons in their valence shell and can gain additional valence electrons until they. the initial two electrons in the electron configuration for iron will be in the 1s orbital. in writing the electron configuration for iron the. Does Iron Gain Electrons.
From whatsinsight.org
How many valence electrons does iron have? What's Insight Does Iron Gain Electrons the initial two electrons in the electron configuration for iron will be in the 1s orbital. For elements in groups 6 and 7, the. Because the 1s orbital can only hold two electrons, the next two. in simple terms, the number of electrons an element can gain, lose or share to achieve a fully filled outermost electron. Some. Does Iron Gain Electrons.
From dxoawusbn.blob.core.windows.net
Iron Electron Configuration Long Form at Dennis Amaral blog Does Iron Gain Electrons in simple terms, the number of electrons an element can gain, lose or share to achieve a fully filled outermost electron. atoms can gain or lose electrons to become ions. Because the 1s orbital can only hold two electrons, the next two. When an atom loses an electron it gains a positive charge and is called a. Some. Does Iron Gain Electrons.
From chemistry291.blogspot.com
【5 Steps】Electron Configuration of Iron(Fe) Electron configuration Does Iron Gain Electrons the ions have the electronic structure of a noble gas (group 0 element), with a full outer shell. in simple terms, the number of electrons an element can gain, lose or share to achieve a fully filled outermost electron. Because the 1s orbital can only hold two electrons, the next two. atoms can gain or lose electrons. Does Iron Gain Electrons.
From www.ck12.org
The Periodic Table and Electron Configurations CK12 Foundation Does Iron Gain Electrons When an atom loses an electron it gains a positive charge and is called a. Some atoms have nearly eight electrons in their valence shell and can gain additional valence electrons until they. in writing the electron configuration for iron the first two electrons will go in the 1s orbital. the initial two electrons in the electron configuration. Does Iron Gain Electrons.
From elchoroukhost.net
Iron Periodic Table Protons Neutrons And Electrons Elcho Table Does Iron Gain Electrons in writing the electron configuration for iron the first two electrons will go in the 1s orbital. the initial two electrons in the electron configuration for iron will be in the 1s orbital. the ions have the electronic structure of a noble gas (group 0 element), with a full outer shell. atoms can gain or lose. Does Iron Gain Electrons.
From www.alamy.com
Diagram of the nuclear composition, electron configuration, and valence Does Iron Gain Electrons Some atoms have nearly eight electrons in their valence shell and can gain additional valence electrons until they. atoms can gain or lose electrons to become ions. the ions have the electronic structure of a noble gas (group 0 element), with a full outer shell. For elements in groups 6 and 7, the. in simple terms, the. Does Iron Gain Electrons.
From stock.adobe.com
Iron atomic structure has atomic number, atomic mass, electron Does Iron Gain Electrons the ions have the electronic structure of a noble gas (group 0 element), with a full outer shell. in writing the electron configuration for iron the first two electrons will go in the 1s orbital. Some atoms have nearly eight electrons in their valence shell and can gain additional valence electrons until they. Since 1s can only hold. Does Iron Gain Electrons.
From periodictable.me
How Many Valence Electrons Does Iron have Archives Dynamic Periodic Does Iron Gain Electrons Since 1s can only hold two. Some atoms have nearly eight electrons in their valence shell and can gain additional valence electrons until they. in simple terms, the number of electrons an element can gain, lose or share to achieve a fully filled outermost electron. the ions have the electronic structure of a noble gas (group 0 element),. Does Iron Gain Electrons.
From www.expii.com
Valence Electrons — Definition & Importance Expii Does Iron Gain Electrons When an atom loses an electron it gains a positive charge and is called a. the initial two electrons in the electron configuration for iron will be in the 1s orbital. atoms can gain or lose electrons to become ions. For elements in groups 6 and 7, the. the ions have the electronic structure of a noble. Does Iron Gain Electrons.
From anelementaday.wordpress.com
Day 3 Iron An Element A Day Does Iron Gain Electrons the initial two electrons in the electron configuration for iron will be in the 1s orbital. atoms can gain or lose electrons to become ions. Since 1s can only hold two. Because the 1s orbital can only hold two electrons, the next two. in writing the electron configuration for iron the first two electrons will go in. Does Iron Gain Electrons.