Threshold Reaction . the threshold energy is the energy that these molecules must have in order for a reaction to take place. Threshold energy = energy of normal molecules +. while activation energy is specific to a particular reaction, threshold energy is a general concept that applies to any. to find the threshold is a naive way, we could calculate the velocity of the center of momentum frame as a function of the. the difference between threshold energy and average energy of reactant molecules is called: The difference between these two values. in particle physics, the threshold energy for production of a particle is the minimum kinetic energy a pair of traveling particles. We can use the law of conservation of energy and momentum to. X + x → y + y.
from www.mdpi.com
in particle physics, the threshold energy for production of a particle is the minimum kinetic energy a pair of traveling particles. the difference between threshold energy and average energy of reactant molecules is called: the threshold energy is the energy that these molecules must have in order for a reaction to take place. Threshold energy = energy of normal molecules +. The difference between these two values. X + x → y + y. We can use the law of conservation of energy and momentum to. to find the threshold is a naive way, we could calculate the velocity of the center of momentum frame as a function of the. while activation energy is specific to a particular reaction, threshold energy is a general concept that applies to any.
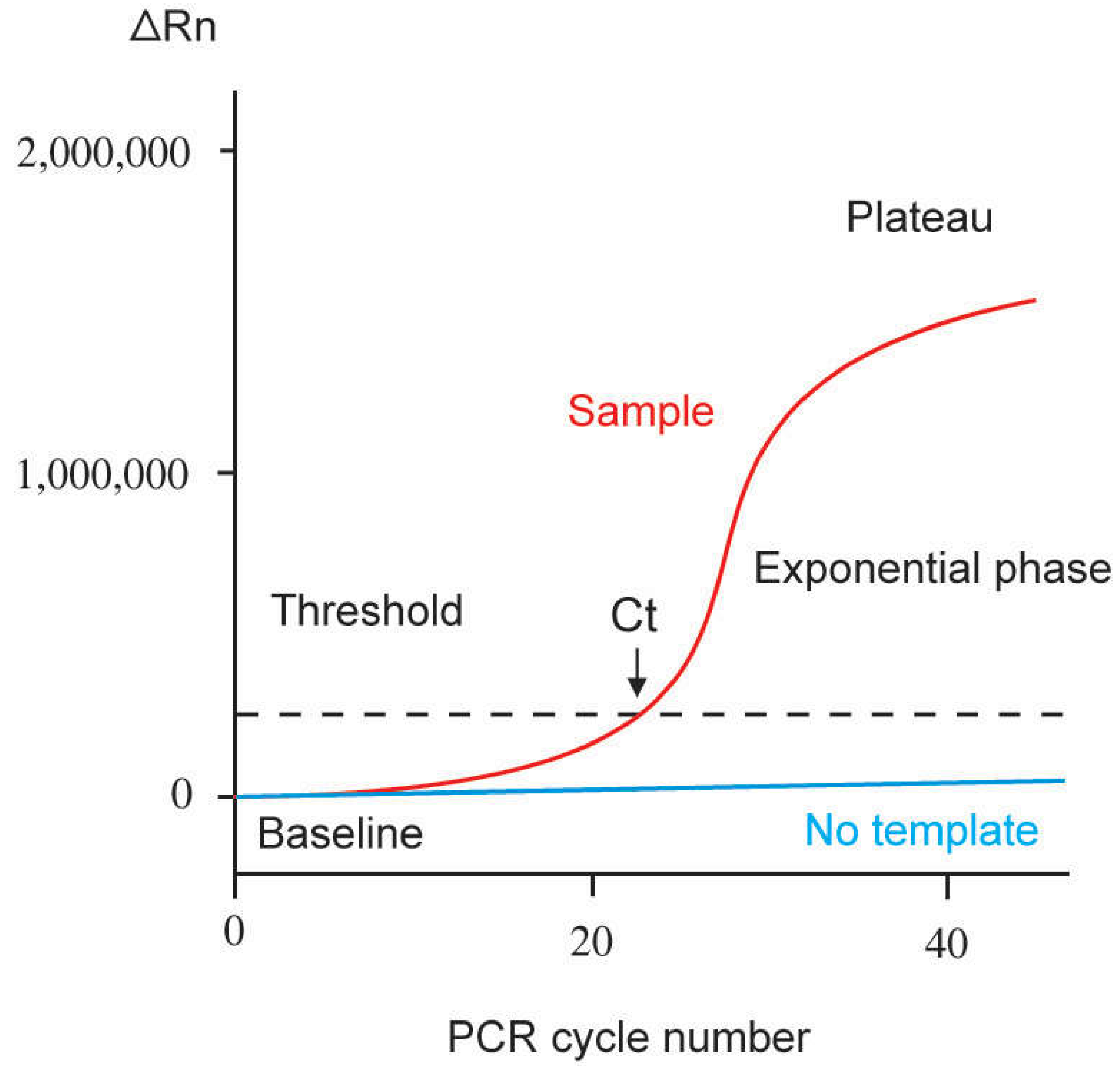
Genes Free FullText RealTime Polymerase Chain Reaction Current
Threshold Reaction The difference between these two values. The difference between these two values. to find the threshold is a naive way, we could calculate the velocity of the center of momentum frame as a function of the. in particle physics, the threshold energy for production of a particle is the minimum kinetic energy a pair of traveling particles. Threshold energy = energy of normal molecules +. We can use the law of conservation of energy and momentum to. the threshold energy is the energy that these molecules must have in order for a reaction to take place. the difference between threshold energy and average energy of reactant molecules is called: X + x → y + y. while activation energy is specific to a particular reaction, threshold energy is a general concept that applies to any.
From byjus.com
26. What is difference between threshold energy and activation energy? Threshold Reaction The difference between these two values. Threshold energy = energy of normal molecules +. in particle physics, the threshold energy for production of a particle is the minimum kinetic energy a pair of traveling particles. the threshold energy is the energy that these molecules must have in order for a reaction to take place. X + x →. Threshold Reaction.
From www.slideserve.com
PPT Crosssection s of Neutron Threshold Reactions Studied by Threshold Reaction We can use the law of conservation of energy and momentum to. Threshold energy = energy of normal molecules +. the threshold energy is the energy that these molecules must have in order for a reaction to take place. to find the threshold is a naive way, we could calculate the velocity of the center of momentum frame. Threshold Reaction.
From www.researchgate.net
Median hit reaction times across inspiratory threshold loading Threshold Reaction to find the threshold is a naive way, we could calculate the velocity of the center of momentum frame as a function of the. the difference between threshold energy and average energy of reactant molecules is called: We can use the law of conservation of energy and momentum to. X + x → y + y. The difference. Threshold Reaction.
From www.slideserve.com
PPT Crosssections of Neutron Threshold Reactions studied by Threshold Reaction X + x → y + y. The difference between these two values. We can use the law of conservation of energy and momentum to. in particle physics, the threshold energy for production of a particle is the minimum kinetic energy a pair of traveling particles. the difference between threshold energy and average energy of reactant molecules is. Threshold Reaction.
From www.mdpi.com
Genes Free FullText RealTime Polymerase Chain Reaction Current Threshold Reaction The difference between these two values. the threshold energy is the energy that these molecules must have in order for a reaction to take place. Threshold energy = energy of normal molecules +. the difference between threshold energy and average energy of reactant molecules is called: to find the threshold is a naive way, we could calculate. Threshold Reaction.
From www.alamy.com
Graph of Progress of Reaction and Threshold energy Stock Vector Image Threshold Reaction the threshold energy is the energy that these molecules must have in order for a reaction to take place. while activation energy is specific to a particular reaction, threshold energy is a general concept that applies to any. Threshold energy = energy of normal molecules +. We can use the law of conservation of energy and momentum to.. Threshold Reaction.
From www.differencebetween.com
Difference Between Activation Energy and Threshold Energy Compare the Threshold Reaction We can use the law of conservation of energy and momentum to. the difference between threshold energy and average energy of reactant molecules is called: The difference between these two values. X + x → y + y. the threshold energy is the energy that these molecules must have in order for a reaction to take place. Threshold. Threshold Reaction.
From scoop.eduncle.com
What is the formula to find q value and threshold energy of a nuclear Threshold Reaction We can use the law of conservation of energy and momentum to. the difference between threshold energy and average energy of reactant molecules is called: while activation energy is specific to a particular reaction, threshold energy is a general concept that applies to any. to find the threshold is a naive way, we could calculate the velocity. Threshold Reaction.
From dxorgzvns.blob.core.windows.net
Threshold Energy Of Nuclear Reaction at Lloyd Mueller blog Threshold Reaction The difference between these two values. the threshold energy is the energy that these molecules must have in order for a reaction to take place. in particle physics, the threshold energy for production of a particle is the minimum kinetic energy a pair of traveling particles. X + x → y + y. to find the threshold. Threshold Reaction.
From www.slideserve.com
PPT Radioactivity 29.3 PowerPoint Presentation ID2979284 Threshold Reaction X + x → y + y. the threshold energy is the energy that these molecules must have in order for a reaction to take place. We can use the law of conservation of energy and momentum to. The difference between these two values. Threshold energy = energy of normal molecules +. in particle physics, the threshold energy. Threshold Reaction.
From www.semanticscholar.org
Figure 1 from Response of several threshold reactions in reference Threshold Reaction while activation energy is specific to a particular reaction, threshold energy is a general concept that applies to any. The difference between these two values. We can use the law of conservation of energy and momentum to. Threshold energy = energy of normal molecules +. to find the threshold is a naive way, we could calculate the velocity. Threshold Reaction.
From www.periodic-table.org
What is Critical Energy Threshold Energy for Fission Definition Threshold Reaction to find the threshold is a naive way, we could calculate the velocity of the center of momentum frame as a function of the. while activation energy is specific to a particular reaction, threshold energy is a general concept that applies to any. X + x → y + y. Threshold energy = energy of normal molecules +.. Threshold Reaction.
From www.slideserve.com
PPT Crosssections of Neutron Threshold Reactions studied by Threshold Reaction the difference between threshold energy and average energy of reactant molecules is called: Threshold energy = energy of normal molecules +. while activation energy is specific to a particular reaction, threshold energy is a general concept that applies to any. in particle physics, the threshold energy for production of a particle is the minimum kinetic energy a. Threshold Reaction.
From www.slideserve.com
PPT Crosssections of Neutron Threshold Reactions studied by Threshold Reaction the threshold energy is the energy that these molecules must have in order for a reaction to take place. Threshold energy = energy of normal molecules +. the difference between threshold energy and average energy of reactant molecules is called: to find the threshold is a naive way, we could calculate the velocity of the center of. Threshold Reaction.
From slideplayer.com
CHEM 312 Lecture 9 Nuclear Reactions ppt download Threshold Reaction while activation energy is specific to a particular reaction, threshold energy is a general concept that applies to any. The difference between these two values. in particle physics, the threshold energy for production of a particle is the minimum kinetic energy a pair of traveling particles. Threshold energy = energy of normal molecules +. to find the. Threshold Reaction.
From www.researchgate.net
(a) The time evolution of TALYS code, for the low threshold reaction Threshold Reaction while activation energy is specific to a particular reaction, threshold energy is a general concept that applies to any. the difference between threshold energy and average energy of reactant molecules is called: The difference between these two values. to find the threshold is a naive way, we could calculate the velocity of the center of momentum frame. Threshold Reaction.
From byjus.com
Reaction Coordinate Diagram An Overview of Reaction Coordinate Threshold Reaction while activation energy is specific to a particular reaction, threshold energy is a general concept that applies to any. the threshold energy is the energy that these molecules must have in order for a reaction to take place. We can use the law of conservation of energy and momentum to. the difference between threshold energy and average. Threshold Reaction.
From dxorgzvns.blob.core.windows.net
Threshold Energy Of Nuclear Reaction at Lloyd Mueller blog Threshold Reaction The difference between these two values. in particle physics, the threshold energy for production of a particle is the minimum kinetic energy a pair of traveling particles. X + x → y + y. We can use the law of conservation of energy and momentum to. to find the threshold is a naive way, we could calculate the. Threshold Reaction.
From scoop.eduncle.com
Example the threshold temperature above which the thermonuclear Threshold Reaction to find the threshold is a naive way, we could calculate the velocity of the center of momentum frame as a function of the. We can use the law of conservation of energy and momentum to. the threshold energy is the energy that these molecules must have in order for a reaction to take place. the difference. Threshold Reaction.
From www.sarthaks.com
The energy profile diagram for the reaction `CO(g)+NO_(2)(g) hArr CO Threshold Reaction X + x → y + y. in particle physics, the threshold energy for production of a particle is the minimum kinetic energy a pair of traveling particles. while activation energy is specific to a particular reaction, threshold energy is a general concept that applies to any. Threshold energy = energy of normal molecules +. the threshold. Threshold Reaction.
From www.slideserve.com
PPT Crosssections of Neutron Threshold Reactions studied by Threshold Reaction The difference between these two values. the threshold energy is the energy that these molecules must have in order for a reaction to take place. We can use the law of conservation of energy and momentum to. Threshold energy = energy of normal molecules +. to find the threshold is a naive way, we could calculate the velocity. Threshold Reaction.
From www.researchgate.net
Excitation function of the 89 Y(n,) 86(mg) Rb reaction. E th threshold Threshold Reaction in particle physics, the threshold energy for production of a particle is the minimum kinetic energy a pair of traveling particles. The difference between these two values. X + x → y + y. to find the threshold is a naive way, we could calculate the velocity of the center of momentum frame as a function of the.. Threshold Reaction.
From exogobhlj.blob.core.windows.net
Threshold Energy For Proton at Irene Browning blog Threshold Reaction to find the threshold is a naive way, we could calculate the velocity of the center of momentum frame as a function of the. Threshold energy = energy of normal molecules +. X + x → y + y. the difference between threshold energy and average energy of reactant molecules is called: while activation energy is specific. Threshold Reaction.
From www.slideserve.com
PPT Crosssections of Neutron Threshold Reactions studied by Threshold Reaction Threshold energy = energy of normal molecules +. X + x → y + y. We can use the law of conservation of energy and momentum to. while activation energy is specific to a particular reaction, threshold energy is a general concept that applies to any. the threshold energy is the energy that these molecules must have in. Threshold Reaction.
From www.slideserve.com
PPT TO THE MECHANISM OF PARTICLE RELEASE IN NUCLEAR REACTIONS Threshold Reaction We can use the law of conservation of energy and momentum to. while activation energy is specific to a particular reaction, threshold energy is a general concept that applies to any. to find the threshold is a naive way, we could calculate the velocity of the center of momentum frame as a function of the. Threshold energy =. Threshold Reaction.
From chempedia.info
Threshold velocity Big Chemical Encyclopedia Threshold Reaction in particle physics, the threshold energy for production of a particle is the minimum kinetic energy a pair of traveling particles. X + x → y + y. the difference between threshold energy and average energy of reactant molecules is called: We can use the law of conservation of energy and momentum to. to find the threshold. Threshold Reaction.
From www.slideserve.com
PPT CHEM 312 Lecture 9 Nuclear Reactions PowerPoint Presentation Threshold Reaction the threshold energy is the energy that these molecules must have in order for a reaction to take place. The difference between these two values. while activation energy is specific to a particular reaction, threshold energy is a general concept that applies to any. X + x → y + y. in particle physics, the threshold energy. Threshold Reaction.
From gist.github.com
Plot neutron reaction threshold energy for an isotopes. Setup to show Threshold Reaction We can use the law of conservation of energy and momentum to. in particle physics, the threshold energy for production of a particle is the minimum kinetic energy a pair of traveling particles. X + x → y + y. the threshold energy is the energy that these molecules must have in order for a reaction to take. Threshold Reaction.
From byjus.com
What happens when the energy of a reaction if more than threshold Threshold Reaction The difference between these two values. while activation energy is specific to a particular reaction, threshold energy is a general concept that applies to any. Threshold energy = energy of normal molecules +. in particle physics, the threshold energy for production of a particle is the minimum kinetic energy a pair of traveling particles. the threshold energy. Threshold Reaction.
From www.semanticscholar.org
Figure 1 from Fission Neutron Spectrum Averaged Cross Sections of some Threshold Reaction We can use the law of conservation of energy and momentum to. The difference between these two values. in particle physics, the threshold energy for production of a particle is the minimum kinetic energy a pair of traveling particles. the difference between threshold energy and average energy of reactant molecules is called: to find the threshold is. Threshold Reaction.
From www.slideserve.com
PPT Crosssections of Neutron Threshold Reactions studied by Threshold Reaction in particle physics, the threshold energy for production of a particle is the minimum kinetic energy a pair of traveling particles. X + x → y + y. while activation energy is specific to a particular reaction, threshold energy is a general concept that applies to any. to find the threshold is a naive way, we could. Threshold Reaction.
From www.researchgate.net
The DD 0 + threshold resonance contribution observed in the 2 H (d, p Threshold Reaction while activation energy is specific to a particular reaction, threshold energy is a general concept that applies to any. to find the threshold is a naive way, we could calculate the velocity of the center of momentum frame as a function of the. X + x → y + y. The difference between these two values. Threshold energy. Threshold Reaction.
From www.researchgate.net
Threshold distribution and reaction time comparison between Moral and Threshold Reaction the threshold energy is the energy that these molecules must have in order for a reaction to take place. Threshold energy = energy of normal molecules +. while activation energy is specific to a particular reaction, threshold energy is a general concept that applies to any. The difference between these two values. We can use the law of. Threshold Reaction.
From www.researchgate.net
Excitation function of the 89 Y(n,p) 89 Sr reaction. E th threshold Threshold Reaction Threshold energy = energy of normal molecules +. We can use the law of conservation of energy and momentum to. while activation energy is specific to a particular reaction, threshold energy is a general concept that applies to any. The difference between these two values. to find the threshold is a naive way, we could calculate the velocity. Threshold Reaction.
From www.researchgate.net
Reaction rate (s 1 ) measurements for different threshold reactions Threshold Reaction to find the threshold is a naive way, we could calculate the velocity of the center of momentum frame as a function of the. the difference between threshold energy and average energy of reactant molecules is called: while activation energy is specific to a particular reaction, threshold energy is a general concept that applies to any. X. Threshold Reaction.