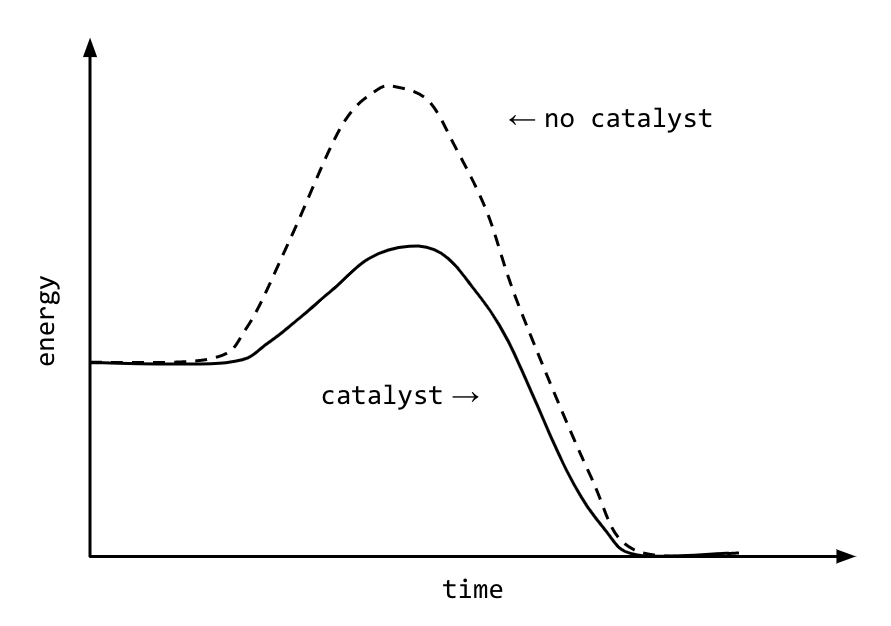
Catalyst Chemistry Graph . It assumes that you are already familiar with. Only a very small mass of catalyst is needed to increase the rate of a reaction. By the end of this section, you will be able to: Different substances catalyse different reactions. This graph compares the reaction coordinates for catalyzed and uncatalyzed alkene hydrogenation. A catalyst is a substance which speeds up a reaction, but is chemically unchanged at the end of the reaction. The effect of catalysts on reaction rates. However, not all reactions have suitable catalysts. Catalysts function by providing an alternate. When the reaction has finished, you would have. This page describes and explains the way that adding a catalyst affects the rate of a reaction. Explain the function of a catalyst in terms of reaction mechanisms and. It assumes familiarity with basic concepts in the collision theory of reaction rates, and with the maxwell. This page explains how adding a catalyst affects the rate of a reaction.
from nesslabs.com
This graph compares the reaction coordinates for catalyzed and uncatalyzed alkene hydrogenation. A catalyst is a substance which speeds up a reaction, but is chemically unchanged at the end of the reaction. It assumes familiarity with basic concepts in the collision theory of reaction rates, and with the maxwell. Only a very small mass of catalyst is needed to increase the rate of a reaction. The effect of catalysts on reaction rates. It assumes that you are already familiar with. Catalysts function by providing an alternate. Explain the function of a catalyst in terms of reaction mechanisms and. This page describes and explains the way that adding a catalyst affects the rate of a reaction. By the end of this section, you will be able to:
Activation energy the chemistry of getting started Ness Labs
Catalyst Chemistry Graph Different substances catalyse different reactions. It assumes familiarity with basic concepts in the collision theory of reaction rates, and with the maxwell. It assumes that you are already familiar with. When the reaction has finished, you would have. A catalyst is a substance which speeds up a reaction, but is chemically unchanged at the end of the reaction. The effect of catalysts on reaction rates. This graph compares the reaction coordinates for catalyzed and uncatalyzed alkene hydrogenation. This page describes and explains the way that adding a catalyst affects the rate of a reaction. This page explains how adding a catalyst affects the rate of a reaction. Catalysts function by providing an alternate. However, not all reactions have suitable catalysts. Different substances catalyse different reactions. Only a very small mass of catalyst is needed to increase the rate of a reaction. By the end of this section, you will be able to: Explain the function of a catalyst in terms of reaction mechanisms and.
From www.organicchemistrytutor.com
Reaction Coordinate Diagram Archives — Organic Chemistry Tutor Catalyst Chemistry Graph Explain the function of a catalyst in terms of reaction mechanisms and. This graph compares the reaction coordinates for catalyzed and uncatalyzed alkene hydrogenation. Different substances catalyse different reactions. By the end of this section, you will be able to: However, not all reactions have suitable catalysts. This page describes and explains the way that adding a catalyst affects the. Catalyst Chemistry Graph.
From courses.lumenlearning.com
Factors Affecting Reaction Rates Chemistry Catalyst Chemistry Graph Different substances catalyse different reactions. When the reaction has finished, you would have. This graph compares the reaction coordinates for catalyzed and uncatalyzed alkene hydrogenation. Only a very small mass of catalyst is needed to increase the rate of a reaction. It assumes that you are already familiar with. By the end of this section, you will be able to:. Catalyst Chemistry Graph.
From www.chemistrystudent.com
Boltzmann Distribution Curves (ALevel) ChemistryStudent Catalyst Chemistry Graph It assumes familiarity with basic concepts in the collision theory of reaction rates, and with the maxwell. When the reaction has finished, you would have. A catalyst is a substance which speeds up a reaction, but is chemically unchanged at the end of the reaction. Explain the function of a catalyst in terms of reaction mechanisms and. Different substances catalyse. Catalyst Chemistry Graph.
From sciencenotes.org
What Is a Catalyst? Understand Catalysis Catalyst Chemistry Graph Explain the function of a catalyst in terms of reaction mechanisms and. It assumes that you are already familiar with. Different substances catalyse different reactions. By the end of this section, you will be able to: However, not all reactions have suitable catalysts. Catalysts function by providing an alternate. This page describes and explains the way that adding a catalyst. Catalyst Chemistry Graph.
From chem.libretexts.org
Catalytic Hydrogenation of Alkenes Chemistry LibreTexts Catalyst Chemistry Graph Different substances catalyse different reactions. It assumes familiarity with basic concepts in the collision theory of reaction rates, and with the maxwell. Catalysts function by providing an alternate. This page describes and explains the way that adding a catalyst affects the rate of a reaction. This graph compares the reaction coordinates for catalyzed and uncatalyzed alkene hydrogenation. By the end. Catalyst Chemistry Graph.
From pressbooks.bccampus.ca
4.6 Catalysis Chemistry for Chemical Engineers Catalyst Chemistry Graph However, not all reactions have suitable catalysts. When the reaction has finished, you would have. This graph compares the reaction coordinates for catalyzed and uncatalyzed alkene hydrogenation. Only a very small mass of catalyst is needed to increase the rate of a reaction. Different substances catalyse different reactions. The effect of catalysts on reaction rates. It assumes familiarity with basic. Catalyst Chemistry Graph.
From schoolbag.info
A catalyst speeds up a reaction by providing the reactants with an Catalyst Chemistry Graph It assumes that you are already familiar with. Catalysts function by providing an alternate. It assumes familiarity with basic concepts in the collision theory of reaction rates, and with the maxwell. This page explains how adding a catalyst affects the rate of a reaction. By the end of this section, you will be able to: Explain the function of a. Catalyst Chemistry Graph.
From circuitdbplastered.z13.web.core.windows.net
Reaction Energy Diagram With Catalyst Catalyst Chemistry Graph It assumes familiarity with basic concepts in the collision theory of reaction rates, and with the maxwell. Only a very small mass of catalyst is needed to increase the rate of a reaction. It assumes that you are already familiar with. When the reaction has finished, you would have. By the end of this section, you will be able to:. Catalyst Chemistry Graph.
From lavelle.chem.ucla.edu
Catalyst effect on energy diagram CHEMISTRY COMMUNITY Catalyst Chemistry Graph Explain the function of a catalyst in terms of reaction mechanisms and. Only a very small mass of catalyst is needed to increase the rate of a reaction. By the end of this section, you will be able to: Different substances catalyse different reactions. It assumes familiarity with basic concepts in the collision theory of reaction rates, and with the. Catalyst Chemistry Graph.
From www.researchgate.net
1 Schematic illustration of a catalytic process showing "A" and "B Catalyst Chemistry Graph Different substances catalyse different reactions. The effect of catalysts on reaction rates. It assumes that you are already familiar with. A catalyst is a substance which speeds up a reaction, but is chemically unchanged at the end of the reaction. Catalysts function by providing an alternate. This page describes and explains the way that adding a catalyst affects the rate. Catalyst Chemistry Graph.
From studymind.co.uk
Catalysts (GCSE Chemistry) Study Mind Catalyst Chemistry Graph It assumes that you are already familiar with. Explain the function of a catalyst in terms of reaction mechanisms and. The effect of catalysts on reaction rates. Only a very small mass of catalyst is needed to increase the rate of a reaction. By the end of this section, you will be able to: When the reaction has finished, you. Catalyst Chemistry Graph.
From www.linstitute.net
Edexcel IGCSE Chemistry 复习笔记 3.3.3 The Position of Equilibrium Catalyst Chemistry Graph This page describes and explains the way that adding a catalyst affects the rate of a reaction. A catalyst is a substance which speeds up a reaction, but is chemically unchanged at the end of the reaction. When the reaction has finished, you would have. However, not all reactions have suitable catalysts. By the end of this section, you will. Catalyst Chemistry Graph.
From www.researchgate.net
Reaction coordinate diagram showing the working principle of a catalyst Catalyst Chemistry Graph This page explains how adding a catalyst affects the rate of a reaction. It assumes that you are already familiar with. When the reaction has finished, you would have. Explain the function of a catalyst in terms of reaction mechanisms and. Catalysts function by providing an alternate. A catalyst is a substance which speeds up a reaction, but is chemically. Catalyst Chemistry Graph.
From derekcarrsavvy-chemist.blogspot.com
savvychemist GCSE OCR Gateway Chemistry C5.2 fi Catalysis and catalysts Catalyst Chemistry Graph However, not all reactions have suitable catalysts. This page explains how adding a catalyst affects the rate of a reaction. This page describes and explains the way that adding a catalyst affects the rate of a reaction. The effect of catalysts on reaction rates. Different substances catalyse different reactions. This graph compares the reaction coordinates for catalyzed and uncatalyzed alkene. Catalyst Chemistry Graph.
From wou.edu
Chapter 7 Catalytic Mechanisms of Enzymes Chemistry Catalyst Chemistry Graph It assumes familiarity with basic concepts in the collision theory of reaction rates, and with the maxwell. A catalyst is a substance which speeds up a reaction, but is chemically unchanged at the end of the reaction. Only a very small mass of catalyst is needed to increase the rate of a reaction. Catalysts function by providing an alternate. This. Catalyst Chemistry Graph.
From 2012books.lardbucket.org
Catalysis Catalyst Chemistry Graph This graph compares the reaction coordinates for catalyzed and uncatalyzed alkene hydrogenation. It assumes familiarity with basic concepts in the collision theory of reaction rates, and with the maxwell. This page describes and explains the way that adding a catalyst affects the rate of a reaction. By the end of this section, you will be able to: Catalysts function by. Catalyst Chemistry Graph.
From philschatz.com
Catalysis · Chemistry Catalyst Chemistry Graph Different substances catalyse different reactions. When the reaction has finished, you would have. This graph compares the reaction coordinates for catalyzed and uncatalyzed alkene hydrogenation. Only a very small mass of catalyst is needed to increase the rate of a reaction. It assumes familiarity with basic concepts in the collision theory of reaction rates, and with the maxwell. By the. Catalyst Chemistry Graph.
From diagramlisthavens.z21.web.core.windows.net
Reaction Coordinate Diagram With Catalyst Catalyst Chemistry Graph This page explains how adding a catalyst affects the rate of a reaction. This page describes and explains the way that adding a catalyst affects the rate of a reaction. Only a very small mass of catalyst is needed to increase the rate of a reaction. The effect of catalysts on reaction rates. However, not all reactions have suitable catalysts.. Catalyst Chemistry Graph.
From www.ck12.org
Catalysts Example 1 ( Video ) Chemistry CK12 Foundation Catalyst Chemistry Graph This page explains how adding a catalyst affects the rate of a reaction. A catalyst is a substance which speeds up a reaction, but is chemically unchanged at the end of the reaction. It assumes that you are already familiar with. Different substances catalyse different reactions. Only a very small mass of catalyst is needed to increase the rate of. Catalyst Chemistry Graph.
From unacademy.com
Positive Catalysts Catalyst Chemistry Graph However, not all reactions have suitable catalysts. When the reaction has finished, you would have. It assumes familiarity with basic concepts in the collision theory of reaction rates, and with the maxwell. A catalyst is a substance which speeds up a reaction, but is chemically unchanged at the end of the reaction. This graph compares the reaction coordinates for catalyzed. Catalyst Chemistry Graph.
From www.researchgate.net
Effect of catalyst on energy diagram profile. Download Scientific Diagram Catalyst Chemistry Graph It assumes that you are already familiar with. This page explains how adding a catalyst affects the rate of a reaction. A catalyst is a substance which speeds up a reaction, but is chemically unchanged at the end of the reaction. Explain the function of a catalyst in terms of reaction mechanisms and. This graph compares the reaction coordinates for. Catalyst Chemistry Graph.
From www.vedantu.com
Le Chatelier’s Principle Laws, Principle Example and FAQs for JEE Catalyst Chemistry Graph This page explains how adding a catalyst affects the rate of a reaction. It assumes familiarity with basic concepts in the collision theory of reaction rates, and with the maxwell. This page describes and explains the way that adding a catalyst affects the rate of a reaction. The effect of catalysts on reaction rates. A catalyst is a substance which. Catalyst Chemistry Graph.
From www.chemistrylearner.com
Activation Energy Definition, Formula, and Graph Catalyst Chemistry Graph The effect of catalysts on reaction rates. This graph compares the reaction coordinates for catalyzed and uncatalyzed alkene hydrogenation. It assumes familiarity with basic concepts in the collision theory of reaction rates, and with the maxwell. Only a very small mass of catalyst is needed to increase the rate of a reaction. This page describes and explains the way that. Catalyst Chemistry Graph.
From www.thoughtco.com
Catalysis Definition in Chemistry Catalyst Chemistry Graph A catalyst is a substance which speeds up a reaction, but is chemically unchanged at the end of the reaction. When the reaction has finished, you would have. This page explains how adding a catalyst affects the rate of a reaction. The effect of catalysts on reaction rates. This page describes and explains the way that adding a catalyst affects. Catalyst Chemistry Graph.
From www.chemengonline.com
Catalysis Fundamentals Chemical Engineering Page 1 Catalyst Chemistry Graph Only a very small mass of catalyst is needed to increase the rate of a reaction. It assumes familiarity with basic concepts in the collision theory of reaction rates, and with the maxwell. It assumes that you are already familiar with. This page describes and explains the way that adding a catalyst affects the rate of a reaction. When the. Catalyst Chemistry Graph.
From www.i-ciencias.com
físicoquímica ¿Que diagrama muestra el efecto de la Catalyst Chemistry Graph Explain the function of a catalyst in terms of reaction mechanisms and. Catalysts function by providing an alternate. It assumes that you are already familiar with. However, not all reactions have suitable catalysts. A catalyst is a substance which speeds up a reaction, but is chemically unchanged at the end of the reaction. The effect of catalysts on reaction rates.. Catalyst Chemistry Graph.
From nesslabs.com
Activation energy the chemistry of getting started Ness Labs Catalyst Chemistry Graph Different substances catalyse different reactions. This page describes and explains the way that adding a catalyst affects the rate of a reaction. Catalysts function by providing an alternate. By the end of this section, you will be able to: The effect of catalysts on reaction rates. However, not all reactions have suitable catalysts. This graph compares the reaction coordinates for. Catalyst Chemistry Graph.
From courses.lumenlearning.com
Catalysis Chemistry Catalyst Chemistry Graph It assumes that you are already familiar with. When the reaction has finished, you would have. A catalyst is a substance which speeds up a reaction, but is chemically unchanged at the end of the reaction. However, not all reactions have suitable catalysts. This graph compares the reaction coordinates for catalyzed and uncatalyzed alkene hydrogenation. Only a very small mass. Catalyst Chemistry Graph.
From www.expii.com
Catalysts (Enzymes) — Overview & Examples Expii Catalyst Chemistry Graph Catalysts function by providing an alternate. This graph compares the reaction coordinates for catalyzed and uncatalyzed alkene hydrogenation. Explain the function of a catalyst in terms of reaction mechanisms and. This page describes and explains the way that adding a catalyst affects the rate of a reaction. The effect of catalysts on reaction rates. It assumes that you are already. Catalyst Chemistry Graph.
From infinitylearn.com
A catalyst increases the rate of reaction by Sri Chaitanya Infinity Catalyst Chemistry Graph The effect of catalysts on reaction rates. Explain the function of a catalyst in terms of reaction mechanisms and. It assumes familiarity with basic concepts in the collision theory of reaction rates, and with the maxwell. Catalysts function by providing an alternate. Only a very small mass of catalyst is needed to increase the rate of a reaction. However, not. Catalyst Chemistry Graph.
From www.youtube.com
Equilibrium Graphs grade 12 Catalyst YouTube Catalyst Chemistry Graph When the reaction has finished, you would have. It assumes familiarity with basic concepts in the collision theory of reaction rates, and with the maxwell. This page explains how adding a catalyst affects the rate of a reaction. It assumes that you are already familiar with. By the end of this section, you will be able to: However, not all. Catalyst Chemistry Graph.
From www.researchgate.net
Free energy of activation of uncatalyzed and catalyzed reactions Catalyst Chemistry Graph This graph compares the reaction coordinates for catalyzed and uncatalyzed alkene hydrogenation. A catalyst is a substance which speeds up a reaction, but is chemically unchanged at the end of the reaction. By the end of this section, you will be able to: Explain the function of a catalyst in terms of reaction mechanisms and. This page describes and explains. Catalyst Chemistry Graph.
From mmerevise.co.uk
Catalysts Questions and Revision MME Catalyst Chemistry Graph Explain the function of a catalyst in terms of reaction mechanisms and. By the end of this section, you will be able to: It assumes familiarity with basic concepts in the collision theory of reaction rates, and with the maxwell. This graph compares the reaction coordinates for catalyzed and uncatalyzed alkene hydrogenation. It assumes that you are already familiar with.. Catalyst Chemistry Graph.
From www.studyorgo.com
Energy Diagram Module Series Part Three Intermediates and Rate Catalyst Chemistry Graph This graph compares the reaction coordinates for catalyzed and uncatalyzed alkene hydrogenation. Catalysts function by providing an alternate. The effect of catalysts on reaction rates. When the reaction has finished, you would have. This page describes and explains the way that adding a catalyst affects the rate of a reaction. However, not all reactions have suitable catalysts. A catalyst is. Catalyst Chemistry Graph.
From www.meritnation.com
Illustrate graphically the effect of a catalyst on rate of a reaction Catalyst Chemistry Graph This graph compares the reaction coordinates for catalyzed and uncatalyzed alkene hydrogenation. By the end of this section, you will be able to: However, not all reactions have suitable catalysts. Only a very small mass of catalyst is needed to increase the rate of a reaction. The effect of catalysts on reaction rates. Catalysts function by providing an alternate. Different. Catalyst Chemistry Graph.