Butane Reaction With Water . The alkenes form a homologous series of unsaturated hydrocarbons. This combustion process is crucial for its role as a fuel in various applications. This page allows searching of all reactions involving this species. Unlike the complex transformations of combustion, the halogenation of an alkane appears to be a simple substitution reaction in. 2c₄h₁₀ + 13o₂ → 8co₂ + 10h₂o + heat. Complete combustion (given sufficient oxygen) of any hydrocarbon produces carbon dioxide and water. Butane is highly flammable, easily reacting with oxygen to ignite, creating carbon dioxide, water, and heat. A general reaction search form is also available. In this exciting experiment, we demonstrate the incredible reaction that occurs when butane gas is. It is quite important that you can write. This is called hydration close hydration chemical reaction in which water reacts with a substance., and it needs a temperature of approximately.
from passmyexams.co.uk
2c₄h₁₀ + 13o₂ → 8co₂ + 10h₂o + heat. The alkenes form a homologous series of unsaturated hydrocarbons. Butane is highly flammable, easily reacting with oxygen to ignite, creating carbon dioxide, water, and heat. It is quite important that you can write. A general reaction search form is also available. This combustion process is crucial for its role as a fuel in various applications. Unlike the complex transformations of combustion, the halogenation of an alkane appears to be a simple substitution reaction in. This page allows searching of all reactions involving this species. In this exciting experiment, we demonstrate the incredible reaction that occurs when butane gas is. Complete combustion (given sufficient oxygen) of any hydrocarbon produces carbon dioxide and water.
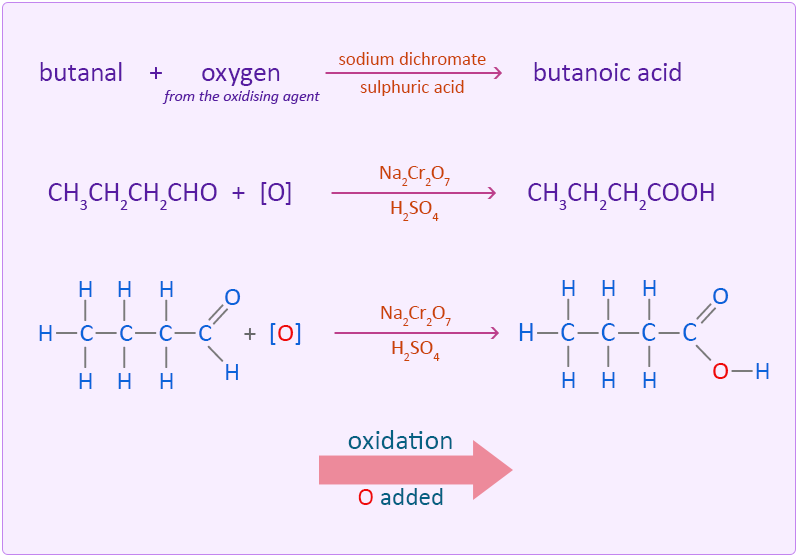
Oxidation of Butanol Easy exam revision notes for GSCE Chemistry
Butane Reaction With Water A general reaction search form is also available. This combustion process is crucial for its role as a fuel in various applications. Complete combustion (given sufficient oxygen) of any hydrocarbon produces carbon dioxide and water. A general reaction search form is also available. 2c₄h₁₀ + 13o₂ → 8co₂ + 10h₂o + heat. This is called hydration close hydration chemical reaction in which water reacts with a substance., and it needs a temperature of approximately. It is quite important that you can write. Butane is highly flammable, easily reacting with oxygen to ignite, creating carbon dioxide, water, and heat. Unlike the complex transformations of combustion, the halogenation of an alkane appears to be a simple substitution reaction in. In this exciting experiment, we demonstrate the incredible reaction that occurs when butane gas is. This page allows searching of all reactions involving this species. The alkenes form a homologous series of unsaturated hydrocarbons.
From www.numerade.com
SOLVED Gaseous butane CH,( CHz CH; will react with gaseous oxygen Butane Reaction With Water This combustion process is crucial for its role as a fuel in various applications. The alkenes form a homologous series of unsaturated hydrocarbons. 2c₄h₁₀ + 13o₂ → 8co₂ + 10h₂o + heat. Butane is highly flammable, easily reacting with oxygen to ignite, creating carbon dioxide, water, and heat. This is called hydration close hydration chemical reaction in which water reacts. Butane Reaction With Water.
From www.numerade.com
SOLVED When butane (C4H10) reacts with oxygen, carbon dioxide and Butane Reaction With Water This is called hydration close hydration chemical reaction in which water reacts with a substance., and it needs a temperature of approximately. 2c₄h₁₀ + 13o₂ → 8co₂ + 10h₂o + heat. Complete combustion (given sufficient oxygen) of any hydrocarbon produces carbon dioxide and water. Unlike the complex transformations of combustion, the halogenation of an alkane appears to be a simple. Butane Reaction With Water.
From www.numerade.com
SOLVED Gaseous butane CH3CH22CH3 will react with gaseous oxygen O2 to Butane Reaction With Water This is called hydration close hydration chemical reaction in which water reacts with a substance., and it needs a temperature of approximately. 2c₄h₁₀ + 13o₂ → 8co₂ + 10h₂o + heat. A general reaction search form is also available. This combustion process is crucial for its role as a fuel in various applications. Unlike the complex transformations of combustion, the. Butane Reaction With Water.
From www.slideserve.com
PPT BALANCING CHEMICAL EQUATIONS PowerPoint Presentation, free Butane Reaction With Water 2c₄h₁₀ + 13o₂ → 8co₂ + 10h₂o + heat. This combustion process is crucial for its role as a fuel in various applications. The alkenes form a homologous series of unsaturated hydrocarbons. A general reaction search form is also available. It is quite important that you can write. Butane is highly flammable, easily reacting with oxygen to ignite, creating carbon. Butane Reaction With Water.
From www.numerade.com
SOLVED Gaseous butane CH3CH22CH3 reacts with gaseous oxygen gas O2 to Butane Reaction With Water In this exciting experiment, we demonstrate the incredible reaction that occurs when butane gas is. Butane is highly flammable, easily reacting with oxygen to ignite, creating carbon dioxide, water, and heat. Unlike the complex transformations of combustion, the halogenation of an alkane appears to be a simple substitution reaction in. It is quite important that you can write. This page. Butane Reaction With Water.
From brainly.in
chemical equation for combustion reactions of the hydrocarbon ,butane Butane Reaction With Water It is quite important that you can write. Butane is highly flammable, easily reacting with oxygen to ignite, creating carbon dioxide, water, and heat. This is called hydration close hydration chemical reaction in which water reacts with a substance., and it needs a temperature of approximately. Unlike the complex transformations of combustion, the halogenation of an alkane appears to be. Butane Reaction With Water.
From melscience.com
Features of the dehydrogenation process of butane MEL Chemistry Butane Reaction With Water In this exciting experiment, we demonstrate the incredible reaction that occurs when butane gas is. A general reaction search form is also available. Unlike the complex transformations of combustion, the halogenation of an alkane appears to be a simple substitution reaction in. Complete combustion (given sufficient oxygen) of any hydrocarbon produces carbon dioxide and water. This page allows searching of. Butane Reaction With Water.
From www.nagwa.com
Question Video Determining the Number of Products Expected from the Butane Reaction With Water This combustion process is crucial for its role as a fuel in various applications. In this exciting experiment, we demonstrate the incredible reaction that occurs when butane gas is. A general reaction search form is also available. Unlike the complex transformations of combustion, the halogenation of an alkane appears to be a simple substitution reaction in. This page allows searching. Butane Reaction With Water.
From www.youtube.com
Ap Chemistry Molecular Mass of Butane Lab Explaination YouTube Butane Reaction With Water The alkenes form a homologous series of unsaturated hydrocarbons. This combustion process is crucial for its role as a fuel in various applications. Butane is highly flammable, easily reacting with oxygen to ignite, creating carbon dioxide, water, and heat. This page allows searching of all reactions involving this species. 2c₄h₁₀ + 13o₂ → 8co₂ + 10h₂o + heat. Unlike the. Butane Reaction With Water.
From questions.kunduz.com
Gaseous butane (CH; (CH2),CH3) will react Organic Chemistry Butane Reaction With Water This is called hydration close hydration chemical reaction in which water reacts with a substance., and it needs a temperature of approximately. The alkenes form a homologous series of unsaturated hydrocarbons. 2c₄h₁₀ + 13o₂ → 8co₂ + 10h₂o + heat. It is quite important that you can write. In this exciting experiment, we demonstrate the incredible reaction that occurs when. Butane Reaction With Water.
From www.numerade.com
SOLVED Gaseous butane (CH3CH22CH3) reacts with gaseous oxygen gas (O2 Butane Reaction With Water It is quite important that you can write. In this exciting experiment, we demonstrate the incredible reaction that occurs when butane gas is. This page allows searching of all reactions involving this species. This combustion process is crucial for its role as a fuel in various applications. 2c₄h₁₀ + 13o₂ → 8co₂ + 10h₂o + heat. Complete combustion (given sufficient. Butane Reaction With Water.
From www.numerade.com
SOLVED write a balanced equation showing the reaction of butane with Butane Reaction With Water In this exciting experiment, we demonstrate the incredible reaction that occurs when butane gas is. It is quite important that you can write. 2c₄h₁₀ + 13o₂ → 8co₂ + 10h₂o + heat. The alkenes form a homologous series of unsaturated hydrocarbons. This page allows searching of all reactions involving this species. Complete combustion (given sufficient oxygen) of any hydrocarbon produces. Butane Reaction With Water.
From www.wou.edu
Chapter 6 Quantities in Chemical Reactions Chemistry Butane Reaction With Water This page allows searching of all reactions involving this species. A general reaction search form is also available. In this exciting experiment, we demonstrate the incredible reaction that occurs when butane gas is. This combustion process is crucial for its role as a fuel in various applications. Complete combustion (given sufficient oxygen) of any hydrocarbon produces carbon dioxide and water.. Butane Reaction With Water.
From www.numerade.com
SOLVED Gaseous butane will react with gaseous oxygen to produce Butane Reaction With Water It is quite important that you can write. This is called hydration close hydration chemical reaction in which water reacts with a substance., and it needs a temperature of approximately. A general reaction search form is also available. The alkenes form a homologous series of unsaturated hydrocarbons. This combustion process is crucial for its role as a fuel in various. Butane Reaction With Water.
From www.chegg.com
Solved Gaseous butane (CH3(CH2), CH3 will react with gaseous Butane Reaction With Water In this exciting experiment, we demonstrate the incredible reaction that occurs when butane gas is. It is quite important that you can write. The alkenes form a homologous series of unsaturated hydrocarbons. 2c₄h₁₀ + 13o₂ → 8co₂ + 10h₂o + heat. Butane is highly flammable, easily reacting with oxygen to ignite, creating carbon dioxide, water, and heat. This page allows. Butane Reaction With Water.
From www.youtube.com
Butanoic Acid + Propanol = ESTER + water YouTube Butane Reaction With Water This page allows searching of all reactions involving this species. This is called hydration close hydration chemical reaction in which water reacts with a substance., and it needs a temperature of approximately. Butane is highly flammable, easily reacting with oxygen to ignite, creating carbon dioxide, water, and heat. 2c₄h₁₀ + 13o₂ → 8co₂ + 10h₂o + heat. Unlike the complex. Butane Reaction With Water.
From www.chegg.com
Solved Gaseous butane (CH3(CH2)2CH3) reacts with gaseous Butane Reaction With Water A general reaction search form is also available. 2c₄h₁₀ + 13o₂ → 8co₂ + 10h₂o + heat. Butane is highly flammable, easily reacting with oxygen to ignite, creating carbon dioxide, water, and heat. Unlike the complex transformations of combustion, the halogenation of an alkane appears to be a simple substitution reaction in. This page allows searching of all reactions involving. Butane Reaction With Water.
From www.coursehero.com
[Solved] Butane is burned with oxygen gas to form carbon dioxide and Butane Reaction With Water Unlike the complex transformations of combustion, the halogenation of an alkane appears to be a simple substitution reaction in. In this exciting experiment, we demonstrate the incredible reaction that occurs when butane gas is. Complete combustion (given sufficient oxygen) of any hydrocarbon produces carbon dioxide and water. This combustion process is crucial for its role as a fuel in various. Butane Reaction With Water.
From passmyexams.co.uk
Oxidation of Butanol Easy exam revision notes for GSCE Chemistry Butane Reaction With Water In this exciting experiment, we demonstrate the incredible reaction that occurs when butane gas is. Unlike the complex transformations of combustion, the halogenation of an alkane appears to be a simple substitution reaction in. A general reaction search form is also available. This page allows searching of all reactions involving this species. This is called hydration close hydration chemical reaction. Butane Reaction With Water.
From www.researchgate.net
Reaction pathway for the selective oxidation of butane over the OMo Butane Reaction With Water The alkenes form a homologous series of unsaturated hydrocarbons. This page allows searching of all reactions involving this species. Unlike the complex transformations of combustion, the halogenation of an alkane appears to be a simple substitution reaction in. This combustion process is crucial for its role as a fuel in various applications. 2c₄h₁₀ + 13o₂ → 8co₂ + 10h₂o +. Butane Reaction With Water.
From solvedlib.com
Gaseous butane(CH3(CH2)2CH3) willreact with gaseous o… SolvedLib Butane Reaction With Water A general reaction search form is also available. The alkenes form a homologous series of unsaturated hydrocarbons. This combustion process is crucial for its role as a fuel in various applications. Unlike the complex transformations of combustion, the halogenation of an alkane appears to be a simple substitution reaction in. Complete combustion (given sufficient oxygen) of any hydrocarbon produces carbon. Butane Reaction With Water.
From kunduz.com
[ANSWERED] The combustion of butane results in the f... Physical Butane Reaction With Water A general reaction search form is also available. Complete combustion (given sufficient oxygen) of any hydrocarbon produces carbon dioxide and water. Unlike the complex transformations of combustion, the halogenation of an alkane appears to be a simple substitution reaction in. The alkenes form a homologous series of unsaturated hydrocarbons. This is called hydration close hydration chemical reaction in which water. Butane Reaction With Water.
From www.toppr.com
1,3 Butadiene on reaction with HBr/water gives primarily 1 bromo 2 Butane Reaction With Water A general reaction search form is also available. Complete combustion (given sufficient oxygen) of any hydrocarbon produces carbon dioxide and water. This page allows searching of all reactions involving this species. 2c₄h₁₀ + 13o₂ → 8co₂ + 10h₂o + heat. This is called hydration close hydration chemical reaction in which water reacts with a substance., and it needs a temperature. Butane Reaction With Water.
From www.coursehero.com
[Solved] Calculate the mass of water produced when 6.67 g of butane Butane Reaction With Water This combustion process is crucial for its role as a fuel in various applications. Butane is highly flammable, easily reacting with oxygen to ignite, creating carbon dioxide, water, and heat. Unlike the complex transformations of combustion, the halogenation of an alkane appears to be a simple substitution reaction in. 2c₄h₁₀ + 13o₂ → 8co₂ + 10h₂o + heat. This page. Butane Reaction With Water.
From byjus.com
How will you convert ethanal into butane 1,3 diol? Butane Reaction With Water This combustion process is crucial for its role as a fuel in various applications. A general reaction search form is also available. This is called hydration close hydration chemical reaction in which water reacts with a substance., and it needs a temperature of approximately. This page allows searching of all reactions involving this species. In this exciting experiment, we demonstrate. Butane Reaction With Water.
From www.numerade.com
SOLVED The balanced chemical equation for the reaction between butane Butane Reaction With Water This page allows searching of all reactions involving this species. Complete combustion (given sufficient oxygen) of any hydrocarbon produces carbon dioxide and water. Butane is highly flammable, easily reacting with oxygen to ignite, creating carbon dioxide, water, and heat. This combustion process is crucial for its role as a fuel in various applications. 2c₄h₁₀ + 13o₂ → 8co₂ + 10h₂o. Butane Reaction With Water.
From www.masterorganicchemistry.com
Bromination of Alkenes Master Organic Chemistry Butane Reaction With Water This page allows searching of all reactions involving this species. This is called hydration close hydration chemical reaction in which water reacts with a substance., and it needs a temperature of approximately. Butane is highly flammable, easily reacting with oxygen to ignite, creating carbon dioxide, water, and heat. Complete combustion (given sufficient oxygen) of any hydrocarbon produces carbon dioxide and. Butane Reaction With Water.
From www.chegg.com
Solved (URGENT) Gaseous butane reacts with gaseous Butane Reaction With Water This is called hydration close hydration chemical reaction in which water reacts with a substance., and it needs a temperature of approximately. 2c₄h₁₀ + 13o₂ → 8co₂ + 10h₂o + heat. Unlike the complex transformations of combustion, the halogenation of an alkane appears to be a simple substitution reaction in. The alkenes form a homologous series of unsaturated hydrocarbons. It. Butane Reaction With Water.
From www.chegg.com
Solved Gaseous butane, CH_3(CH_2)_2CH_3 reacts with gaseous Butane Reaction With Water In this exciting experiment, we demonstrate the incredible reaction that occurs when butane gas is. The alkenes form a homologous series of unsaturated hydrocarbons. Unlike the complex transformations of combustion, the halogenation of an alkane appears to be a simple substitution reaction in. Complete combustion (given sufficient oxygen) of any hydrocarbon produces carbon dioxide and water. A general reaction search. Butane Reaction With Water.
From www.chegg.com
Solved Butane solution was added with bromine water and Butane Reaction With Water Unlike the complex transformations of combustion, the halogenation of an alkane appears to be a simple substitution reaction in. The alkenes form a homologous series of unsaturated hydrocarbons. It is quite important that you can write. This combustion process is crucial for its role as a fuel in various applications. Complete combustion (given sufficient oxygen) of any hydrocarbon produces carbon. Butane Reaction With Water.
From www.numerade.com
SOLVED Gaseous butane will react with gaseous oxygen to produce Butane Reaction With Water Butane is highly flammable, easily reacting with oxygen to ignite, creating carbon dioxide, water, and heat. The alkenes form a homologous series of unsaturated hydrocarbons. 2c₄h₁₀ + 13o₂ → 8co₂ + 10h₂o + heat. This combustion process is crucial for its role as a fuel in various applications. It is quite important that you can write. In this exciting experiment,. Butane Reaction With Water.
From www.doubtnut.com
Butane (C(4)H(10)) gas burns in oxygen to give carbon dioxide and water Butane Reaction With Water A general reaction search form is also available. Butane is highly flammable, easily reacting with oxygen to ignite, creating carbon dioxide, water, and heat. It is quite important that you can write. Complete combustion (given sufficient oxygen) of any hydrocarbon produces carbon dioxide and water. In this exciting experiment, we demonstrate the incredible reaction that occurs when butane gas is.. Butane Reaction With Water.
From www.numerade.com
SOLVED When butane (C4H10) reacts with oxygen, carbon dioxide and Butane Reaction With Water Butane is highly flammable, easily reacting with oxygen to ignite, creating carbon dioxide, water, and heat. 2c₄h₁₀ + 13o₂ → 8co₂ + 10h₂o + heat. This combustion process is crucial for its role as a fuel in various applications. In this exciting experiment, we demonstrate the incredible reaction that occurs when butane gas is. The alkenes form a homologous series. Butane Reaction With Water.
From www.slideserve.com
PPT Substitution Reactions 1 The Sn 2 Reaction The Synthesis of 1 Butane Reaction With Water 2c₄h₁₀ + 13o₂ → 8co₂ + 10h₂o + heat. This page allows searching of all reactions involving this species. Unlike the complex transformations of combustion, the halogenation of an alkane appears to be a simple substitution reaction in. In this exciting experiment, we demonstrate the incredible reaction that occurs when butane gas is. This is called hydration close hydration chemical. Butane Reaction With Water.
From www.coursehero.com
[Solved] Gaseous butane CH3CH22CH3 reacts with gaseous oxygen gas O2 to Butane Reaction With Water In this exciting experiment, we demonstrate the incredible reaction that occurs when butane gas is. It is quite important that you can write. Unlike the complex transformations of combustion, the halogenation of an alkane appears to be a simple substitution reaction in. A general reaction search form is also available. 2c₄h₁₀ + 13o₂ → 8co₂ + 10h₂o + heat. This. Butane Reaction With Water.