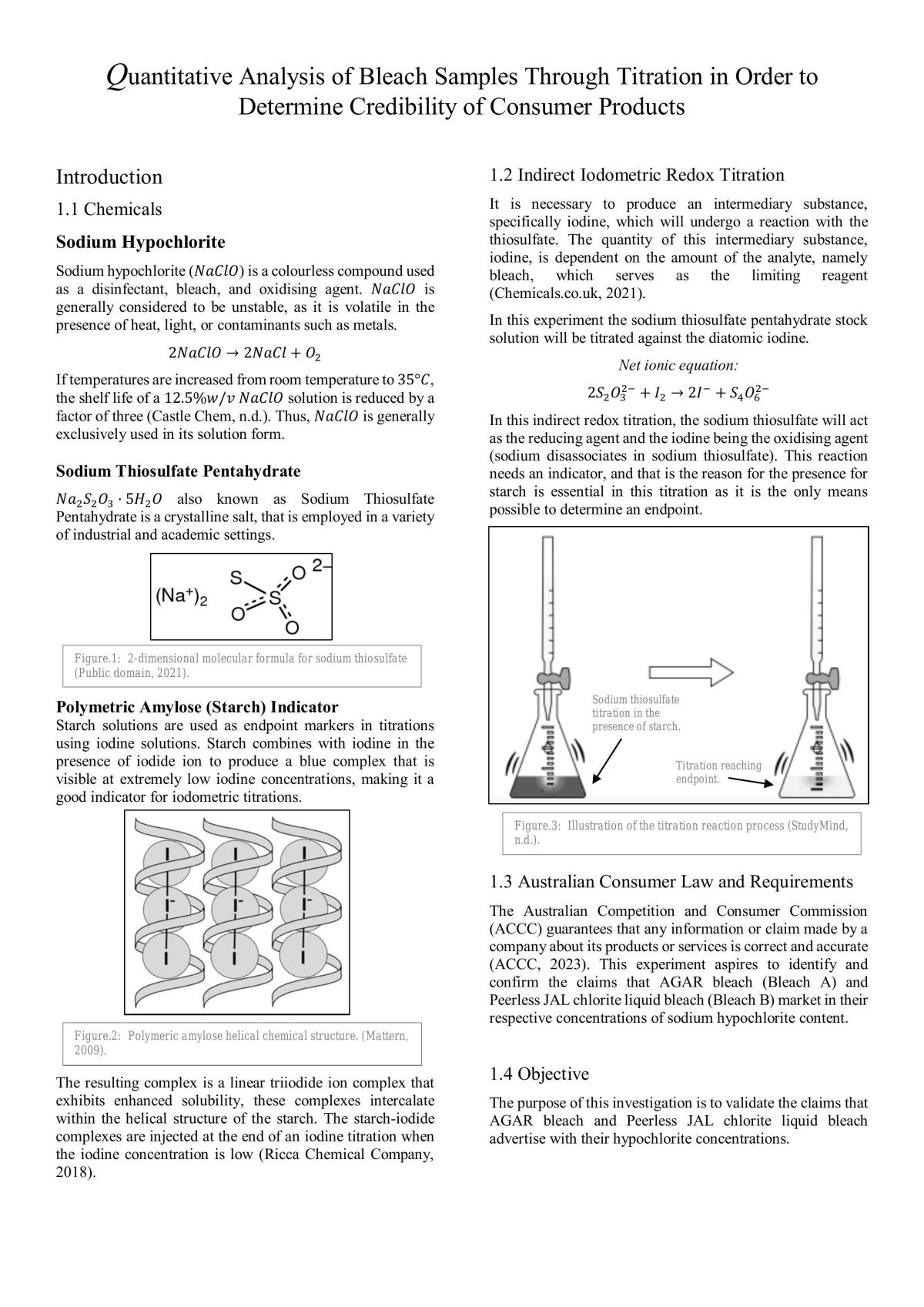
Bleach Titration Lab Report . The most common and successful method for use in high schools. Starch is used to visualize the. The determination of free chlorine in bleach is possible by a redox titration. It is possible to determine the concentration of hypochlorite in bleach in the laboratory using a 2 step process: The oxidizing power (percent naocl) of commercial bleach is determined iodometrically by reacting it with an excess of iodide in acetic. A redox titration is conducted to determine the concentration of sodium hypochlorite in the bleach sample. Using the format specified, prepare a lab report summarizing the data for your thiosulfate standardizations (masses of kio3, volumes of. Each pair of students will titrate one brand of commercial liquid bleach to find the amount of “available chlorine” and the percent, by mass,. The determination of hypochlorite in bleach.
from www.thinkswap.com
The oxidizing power (percent naocl) of commercial bleach is determined iodometrically by reacting it with an excess of iodide in acetic. Using the format specified, prepare a lab report summarizing the data for your thiosulfate standardizations (masses of kio3, volumes of. The determination of free chlorine in bleach is possible by a redox titration. A redox titration is conducted to determine the concentration of sodium hypochlorite in the bleach sample. The determination of hypochlorite in bleach. The most common and successful method for use in high schools. It is possible to determine the concentration of hypochlorite in bleach in the laboratory using a 2 step process: Each pair of students will titrate one brand of commercial liquid bleach to find the amount of “available chlorine” and the percent, by mass,. Starch is used to visualize the.
Chemistry A+ Practical Report Quantitative Analysis of Bleach Samples
Bleach Titration Lab Report Starch is used to visualize the. Starch is used to visualize the. Each pair of students will titrate one brand of commercial liquid bleach to find the amount of “available chlorine” and the percent, by mass,. The oxidizing power (percent naocl) of commercial bleach is determined iodometrically by reacting it with an excess of iodide in acetic. The determination of hypochlorite in bleach. Using the format specified, prepare a lab report summarizing the data for your thiosulfate standardizations (masses of kio3, volumes of. The determination of free chlorine in bleach is possible by a redox titration. It is possible to determine the concentration of hypochlorite in bleach in the laboratory using a 2 step process: A redox titration is conducted to determine the concentration of sodium hypochlorite in the bleach sample. The most common and successful method for use in high schools.
From www.youtube.com
Bleach Titration Calculations Example YouTube Bleach Titration Lab Report The oxidizing power (percent naocl) of commercial bleach is determined iodometrically by reacting it with an excess of iodide in acetic. Using the format specified, prepare a lab report summarizing the data for your thiosulfate standardizations (masses of kio3, volumes of. Each pair of students will titrate one brand of commercial liquid bleach to find the amount of “available chlorine”. Bleach Titration Lab Report.
From www.scribd.com
chemistry lab report titration lab Molar Concentration Mole (Unit) Bleach Titration Lab Report Using the format specified, prepare a lab report summarizing the data for your thiosulfate standardizations (masses of kio3, volumes of. The oxidizing power (percent naocl) of commercial bleach is determined iodometrically by reacting it with an excess of iodide in acetic. The determination of free chlorine in bleach is possible by a redox titration. A redox titration is conducted to. Bleach Titration Lab Report.
From www.numerade.com
SOLVED REPORT SUMMARY Determination of the Amount of Sodium Bleach Titration Lab Report The determination of free chlorine in bleach is possible by a redox titration. The oxidizing power (percent naocl) of commercial bleach is determined iodometrically by reacting it with an excess of iodide in acetic. Each pair of students will titrate one brand of commercial liquid bleach to find the amount of “available chlorine” and the percent, by mass,. It is. Bleach Titration Lab Report.
From www.studocu.com
Bleach Analysis laboratory report Analysis of Hypochlorite in Bleach Titration Lab Report Starch is used to visualize the. The determination of free chlorine in bleach is possible by a redox titration. Each pair of students will titrate one brand of commercial liquid bleach to find the amount of “available chlorine” and the percent, by mass,. A redox titration is conducted to determine the concentration of sodium hypochlorite in the bleach sample. Using. Bleach Titration Lab Report.
From www.chegg.com
Assay of Commercial Bleach Data/Report Sheet Date Bleach Titration Lab Report It is possible to determine the concentration of hypochlorite in bleach in the laboratory using a 2 step process: Using the format specified, prepare a lab report summarizing the data for your thiosulfate standardizations (masses of kio3, volumes of. The most common and successful method for use in high schools. The determination of free chlorine in bleach is possible by. Bleach Titration Lab Report.
From www.youtube.com
Analysis of bleach redox titration lab YouTube Bleach Titration Lab Report Using the format specified, prepare a lab report summarizing the data for your thiosulfate standardizations (masses of kio3, volumes of. The oxidizing power (percent naocl) of commercial bleach is determined iodometrically by reacting it with an excess of iodide in acetic. A redox titration is conducted to determine the concentration of sodium hypochlorite in the bleach sample. Starch is used. Bleach Titration Lab Report.
From www.studocu.com
Bleach Lab Report Lab Warning TT undefined function 32 Ashley Bleach Titration Lab Report It is possible to determine the concentration of hypochlorite in bleach in the laboratory using a 2 step process: A redox titration is conducted to determine the concentration of sodium hypochlorite in the bleach sample. The determination of hypochlorite in bleach. Each pair of students will titrate one brand of commercial liquid bleach to find the amount of “available chlorine”. Bleach Titration Lab Report.
From www.youtube.com
Determination of Percentage () Available Chlorine in Bleaching Powder Bleach Titration Lab Report Starch is used to visualize the. Using the format specified, prepare a lab report summarizing the data for your thiosulfate standardizations (masses of kio3, volumes of. The most common and successful method for use in high schools. Each pair of students will titrate one brand of commercial liquid bleach to find the amount of “available chlorine” and the percent, by. Bleach Titration Lab Report.
From www.docsity.com
Iodometric Titration of Commercial Bleach Lab Reports Chemistry Docsity Bleach Titration Lab Report The determination of hypochlorite in bleach. It is possible to determine the concentration of hypochlorite in bleach in the laboratory using a 2 step process: The determination of free chlorine in bleach is possible by a redox titration. Each pair of students will titrate one brand of commercial liquid bleach to find the amount of “available chlorine” and the percent,. Bleach Titration Lab Report.
From www.chegg.com
Solved Redox Titration Analysis of Bleach lab report Bleach Titration Lab Report Each pair of students will titrate one brand of commercial liquid bleach to find the amount of “available chlorine” and the percent, by mass,. The most common and successful method for use in high schools. The determination of hypochlorite in bleach. A redox titration is conducted to determine the concentration of sodium hypochlorite in the bleach sample. Starch is used. Bleach Titration Lab Report.
From www.chegg.com
Solved Redox Titration Analysis of Bleach lab report Bleach Titration Lab Report Starch is used to visualize the. The determination of hypochlorite in bleach. The oxidizing power (percent naocl) of commercial bleach is determined iodometrically by reacting it with an excess of iodide in acetic. It is possible to determine the concentration of hypochlorite in bleach in the laboratory using a 2 step process: Each pair of students will titrate one brand. Bleach Titration Lab Report.
From studylib.net
Analysis of Commercial Bleach Bleach Titration Lab Report The most common and successful method for use in high schools. A redox titration is conducted to determine the concentration of sodium hypochlorite in the bleach sample. Starch is used to visualize the. The determination of hypochlorite in bleach. Using the format specified, prepare a lab report summarizing the data for your thiosulfate standardizations (masses of kio3, volumes of. The. Bleach Titration Lab Report.
From www.studocu.com
Titration of bleach report template Principles of Chemistry 2 Bleach Titration Lab Report Each pair of students will titrate one brand of commercial liquid bleach to find the amount of “available chlorine” and the percent, by mass,. The determination of hypochlorite in bleach. The most common and successful method for use in high schools. Starch is used to visualize the. It is possible to determine the concentration of hypochlorite in bleach in the. Bleach Titration Lab Report.
From www.thinkswap.com
Chemistry A+ Practical Report Quantitative Analysis of Bleach Samples Bleach Titration Lab Report Starch is used to visualize the. Each pair of students will titrate one brand of commercial liquid bleach to find the amount of “available chlorine” and the percent, by mass,. It is possible to determine the concentration of hypochlorite in bleach in the laboratory using a 2 step process: The oxidizing power (percent naocl) of commercial bleach is determined iodometrically. Bleach Titration Lab Report.
From studylib.net
ANALYSIS OF BLEACH BY THIOSULFATE TITRATION Bleach Titration Lab Report Starch is used to visualize the. The oxidizing power (percent naocl) of commercial bleach is determined iodometrically by reacting it with an excess of iodide in acetic. The most common and successful method for use in high schools. It is possible to determine the concentration of hypochlorite in bleach in the laboratory using a 2 step process: The determination of. Bleach Titration Lab Report.
From www.studocu.com
Bleach notes laboratory report Analysis of Hypochlorite in Bleach Bleach Titration Lab Report Starch is used to visualize the. The determination of hypochlorite in bleach. A redox titration is conducted to determine the concentration of sodium hypochlorite in the bleach sample. It is possible to determine the concentration of hypochlorite in bleach in the laboratory using a 2 step process: The oxidizing power (percent naocl) of commercial bleach is determined iodometrically by reacting. Bleach Titration Lab Report.
From www.chegg.com
Solved REPORT SHEET OxidationReduction Titrations II Bleach Titration Lab Report A redox titration is conducted to determine the concentration of sodium hypochlorite in the bleach sample. The determination of free chlorine in bleach is possible by a redox titration. The most common and successful method for use in high schools. The oxidizing power (percent naocl) of commercial bleach is determined iodometrically by reacting it with an excess of iodide in. Bleach Titration Lab Report.
From www.chegg.com
Solved EXPERIMENT 18 Determination Of BleachA Redox Tit... Bleach Titration Lab Report Using the format specified, prepare a lab report summarizing the data for your thiosulfate standardizations (masses of kio3, volumes of. The oxidizing power (percent naocl) of commercial bleach is determined iodometrically by reacting it with an excess of iodide in acetic. Each pair of students will titrate one brand of commercial liquid bleach to find the amount of “available chlorine”. Bleach Titration Lab Report.
From www.studocu.com
Bleach titration How to write “Determination of Percent Sodium Bleach Titration Lab Report Using the format specified, prepare a lab report summarizing the data for your thiosulfate standardizations (masses of kio3, volumes of. Each pair of students will titrate one brand of commercial liquid bleach to find the amount of “available chlorine” and the percent, by mass,. The determination of free chlorine in bleach is possible by a redox titration. It is possible. Bleach Titration Lab Report.
From www.numerade.com
SOLVED Bleach titration experiment Sodium hypochlorite (Mass = 74.44 Bleach Titration Lab Report Starch is used to visualize the. The determination of hypochlorite in bleach. It is possible to determine the concentration of hypochlorite in bleach in the laboratory using a 2 step process: The determination of free chlorine in bleach is possible by a redox titration. Each pair of students will titrate one brand of commercial liquid bleach to find the amount. Bleach Titration Lab Report.
From www.studocu.com
Name's Copy of Titration Lab Report Name Titration Lab Report Date Bleach Titration Lab Report The oxidizing power (percent naocl) of commercial bleach is determined iodometrically by reacting it with an excess of iodide in acetic. It is possible to determine the concentration of hypochlorite in bleach in the laboratory using a 2 step process: Starch is used to visualize the. The determination of hypochlorite in bleach. The most common and successful method for use. Bleach Titration Lab Report.
From www.vrogue.co
Redox Titration Lab Sheet 1 Docx Redox Titration Lab vrogue.co Bleach Titration Lab Report The oxidizing power (percent naocl) of commercial bleach is determined iodometrically by reacting it with an excess of iodide in acetic. The most common and successful method for use in high schools. Starch is used to visualize the. The determination of free chlorine in bleach is possible by a redox titration. Using the format specified, prepare a lab report summarizing. Bleach Titration Lab Report.
From studylib.net
Analysis of Bleach Bleach Titration Lab Report The oxidizing power (percent naocl) of commercial bleach is determined iodometrically by reacting it with an excess of iodide in acetic. Starch is used to visualize the. The most common and successful method for use in high schools. Each pair of students will titrate one brand of commercial liquid bleach to find the amount of “available chlorine” and the percent,. Bleach Titration Lab Report.
From www.scribd.com
analysis of a commercial bleach lab Titration Redox Bleach Titration Lab Report A redox titration is conducted to determine the concentration of sodium hypochlorite in the bleach sample. It is possible to determine the concentration of hypochlorite in bleach in the laboratory using a 2 step process: Starch is used to visualize the. The determination of hypochlorite in bleach. Using the format specified, prepare a lab report summarizing the data for your. Bleach Titration Lab Report.
From www.chegg.com
Solved EXPERIMENT 18 Determination of BleachA Redox Bleach Titration Lab Report The most common and successful method for use in high schools. It is possible to determine the concentration of hypochlorite in bleach in the laboratory using a 2 step process: Each pair of students will titrate one brand of commercial liquid bleach to find the amount of “available chlorine” and the percent, by mass,. Using the format specified, prepare a. Bleach Titration Lab Report.
From studylib.net
Lab 8 Redox Titration of Bleach Bleach Titration Lab Report A redox titration is conducted to determine the concentration of sodium hypochlorite in the bleach sample. It is possible to determine the concentration of hypochlorite in bleach in the laboratory using a 2 step process: Each pair of students will titrate one brand of commercial liquid bleach to find the amount of “available chlorine” and the percent, by mass,. The. Bleach Titration Lab Report.
From studylib.net
Redox Titration Bleach Titration Lab Report Starch is used to visualize the. Using the format specified, prepare a lab report summarizing the data for your thiosulfate standardizations (masses of kio3, volumes of. The oxidizing power (percent naocl) of commercial bleach is determined iodometrically by reacting it with an excess of iodide in acetic. The most common and successful method for use in high schools. It is. Bleach Titration Lab Report.
From dokumen.tips
(PDF) LAB 1 Redox Titration of Bleach DOKUMEN.TIPS Bleach Titration Lab Report The oxidizing power (percent naocl) of commercial bleach is determined iodometrically by reacting it with an excess of iodide in acetic. The most common and successful method for use in high schools. Using the format specified, prepare a lab report summarizing the data for your thiosulfate standardizations (masses of kio3, volumes of. Each pair of students will titrate one brand. Bleach Titration Lab Report.
From studylib.net
Bleach titration practical exemplar Bleach Titration Lab Report The oxidizing power (percent naocl) of commercial bleach is determined iodometrically by reacting it with an excess of iodide in acetic. Each pair of students will titrate one brand of commercial liquid bleach to find the amount of “available chlorine” and the percent, by mass,. The determination of free chlorine in bleach is possible by a redox titration. Starch is. Bleach Titration Lab Report.
From www.studocu.com
Titration Lab Report Titration Lab Report Objective To find the Bleach Titration Lab Report A redox titration is conducted to determine the concentration of sodium hypochlorite in the bleach sample. The oxidizing power (percent naocl) of commercial bleach is determined iodometrically by reacting it with an excess of iodide in acetic. Using the format specified, prepare a lab report summarizing the data for your thiosulfate standardizations (masses of kio3, volumes of. Starch is used. Bleach Titration Lab Report.
From www.scribd.com
Laboratory Report Chm421 Experiment 6A Analysis of Bleach and Copper Bleach Titration Lab Report Each pair of students will titrate one brand of commercial liquid bleach to find the amount of “available chlorine” and the percent, by mass,. Starch is used to visualize the. The oxidizing power (percent naocl) of commercial bleach is determined iodometrically by reacting it with an excess of iodide in acetic. The determination of free chlorine in bleach is possible. Bleach Titration Lab Report.
From www.chegg.com
Redox Titration Analysis of Bleach lab report Bleach Titration Lab Report A redox titration is conducted to determine the concentration of sodium hypochlorite in the bleach sample. It is possible to determine the concentration of hypochlorite in bleach in the laboratory using a 2 step process: Each pair of students will titrate one brand of commercial liquid bleach to find the amount of “available chlorine” and the percent, by mass,. The. Bleach Titration Lab Report.
From dokumen.tips
(PDF) A Volumetric Analysis (Redox Titration) of Hypochlorite in Bleach Titration Lab Report The determination of hypochlorite in bleach. The determination of free chlorine in bleach is possible by a redox titration. Starch is used to visualize the. A redox titration is conducted to determine the concentration of sodium hypochlorite in the bleach sample. The most common and successful method for use in high schools. Using the format specified, prepare a lab report. Bleach Titration Lab Report.
From www.numerade.com
SOLVED in the lab experiment, Redox Titration Determination of Sodium Bleach Titration Lab Report A redox titration is conducted to determine the concentration of sodium hypochlorite in the bleach sample. The oxidizing power (percent naocl) of commercial bleach is determined iodometrically by reacting it with an excess of iodide in acetic. The determination of free chlorine in bleach is possible by a redox titration. It is possible to determine the concentration of hypochlorite in. Bleach Titration Lab Report.
From www.studocu.com
Analysis of a Commercial Bleach Solution Lab Report Complete the Bleach Titration Lab Report Starch is used to visualize the. Each pair of students will titrate one brand of commercial liquid bleach to find the amount of “available chlorine” and the percent, by mass,. It is possible to determine the concentration of hypochlorite in bleach in the laboratory using a 2 step process: The determination of hypochlorite in bleach. The oxidizing power (percent naocl). Bleach Titration Lab Report.