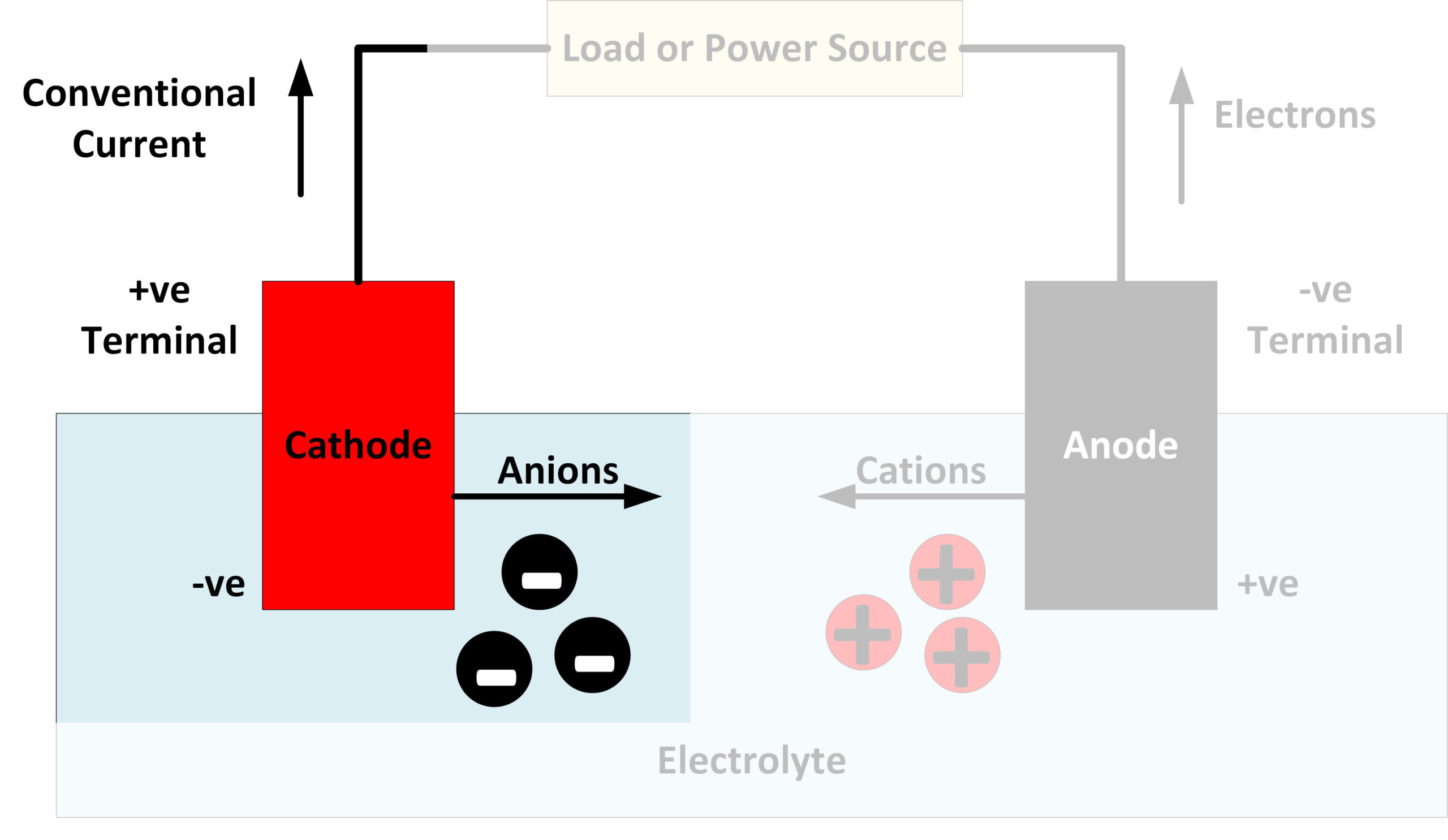
Positive Electrode Color . An electrolytic cell converts electrical energy into chemical energy. In a galvanic (voltaic) cell, the anode is considered negative and the cathode is considered positive. The cathode (electrode in beaker that. The positive electrode is a carbon fibre rod and the negative electrode is an iron nail. Here, the redox reaction is spontaneous and is responsible for the production of electrical energy. Chloride ions produce gaseous chlorine bromide and iodide ions form bromine and iodine respectively which dissolve to form. The negative ions are attracted to the positive electrode. The redox reaction is not spontaneous and electrical energy has to be supplied to initiate the reaction. The electrode attached to the positive terminal of a battery is the positive electrode, or anode., called a cathode close cathode the negative. The pt electrode in the permanganate solution is the cathode; The chloride ions are discharged (giving chlorine) in preference to the hydroxide ions. A small spirit burner provides sufficient heat to melt the lead bromide. The one in the tin solution is the anode. This seems reasonable as the anode is the source of.
from bastatrain.weebly.com
The negative ions are attracted to the positive electrode. An electrolytic cell converts electrical energy into chemical energy. Here, the redox reaction is spontaneous and is responsible for the production of electrical energy. The one in the tin solution is the anode. The pt electrode in the permanganate solution is the cathode; The electrode attached to the positive terminal of a battery is the positive electrode, or anode., called a cathode close cathode the negative. A small spirit burner provides sufficient heat to melt the lead bromide. This seems reasonable as the anode is the source of. Chloride ions produce gaseous chlorine bromide and iodide ions form bromine and iodine respectively which dissolve to form. The redox reaction is not spontaneous and electrical energy has to be supplied to initiate the reaction.
Cathode led positive or negative bastatrain
Positive Electrode Color The pt electrode in the permanganate solution is the cathode; The positive electrode is a carbon fibre rod and the negative electrode is an iron nail. The redox reaction is not spontaneous and electrical energy has to be supplied to initiate the reaction. The cathode (electrode in beaker that. In a galvanic (voltaic) cell, the anode is considered negative and the cathode is considered positive. The negative ions are attracted to the positive electrode. The electrode attached to the positive terminal of a battery is the positive electrode, or anode., called a cathode close cathode the negative. The chloride ions are discharged (giving chlorine) in preference to the hydroxide ions. An electrolytic cell converts electrical energy into chemical energy. The one in the tin solution is the anode. Here, the redox reaction is spontaneous and is responsible for the production of electrical energy. The pt electrode in the permanganate solution is the cathode; Chloride ions produce gaseous chlorine bromide and iodide ions form bromine and iodine respectively which dissolve to form. This seems reasonable as the anode is the source of. A small spirit burner provides sufficient heat to melt the lead bromide.
From www.researchgate.net
Negative and positive electrode materials for LIBs. Blue outlined Positive Electrode Color The one in the tin solution is the anode. The electrode attached to the positive terminal of a battery is the positive electrode, or anode., called a cathode close cathode the negative. The redox reaction is not spontaneous and electrical energy has to be supplied to initiate the reaction. The negative ions are attracted to the positive electrode. This seems. Positive Electrode Color.
From www.alamy.com
Ions movement to negative electrode and positive electrode. 3D Positive Electrode Color The chloride ions are discharged (giving chlorine) in preference to the hydroxide ions. An electrolytic cell converts electrical energy into chemical energy. This seems reasonable as the anode is the source of. The electrode attached to the positive terminal of a battery is the positive electrode, or anode., called a cathode close cathode the negative. The one in the tin. Positive Electrode Color.
From www.fdk.com
Positive electrode material │ FDK's original technology │ R&D │ FDK Positive Electrode Color The redox reaction is not spontaneous and electrical energy has to be supplied to initiate the reaction. Here, the redox reaction is spontaneous and is responsible for the production of electrical energy. In a galvanic (voltaic) cell, the anode is considered negative and the cathode is considered positive. Chloride ions produce gaseous chlorine bromide and iodide ions form bromine and. Positive Electrode Color.
From weldguru.com
TIG Tungsten Electrodes Explained (with Color Chart) Positive Electrode Color The positive electrode is a carbon fibre rod and the negative electrode is an iron nail. The cathode (electrode in beaker that. Chloride ions produce gaseous chlorine bromide and iodide ions form bromine and iodine respectively which dissolve to form. The redox reaction is not spontaneous and electrical energy has to be supplied to initiate the reaction. The negative ions. Positive Electrode Color.
From www.youtube.com
Color Coding of Tungsten Electrodes tungsten electrode tungsten Positive Electrode Color The pt electrode in the permanganate solution is the cathode; The positive electrode is a carbon fibre rod and the negative electrode is an iron nail. An electrolytic cell converts electrical energy into chemical energy. Chloride ions produce gaseous chlorine bromide and iodide ions form bromine and iodine respectively which dissolve to form. In a galvanic (voltaic) cell, the anode. Positive Electrode Color.
From stock.adobe.com
Ions movement to negative electrode and positive electrode Stock Photo Positive Electrode Color This seems reasonable as the anode is the source of. Chloride ions produce gaseous chlorine bromide and iodide ions form bromine and iodine respectively which dissolve to form. The chloride ions are discharged (giving chlorine) in preference to the hydroxide ions. The cathode (electrode in beaker that. The one in the tin solution is the anode. The negative ions are. Positive Electrode Color.
From www.researchgate.net
Figure1. Diagrams of positive electrode active mass of the Positive Electrode Color The chloride ions are discharged (giving chlorine) in preference to the hydroxide ions. An electrolytic cell converts electrical energy into chemical energy. The negative ions are attracted to the positive electrode. The positive electrode is a carbon fibre rod and the negative electrode is an iron nail. Chloride ions produce gaseous chlorine bromide and iodide ions form bromine and iodine. Positive Electrode Color.
From mungfali.com
Types Of Electrodes Positive Electrode Color The electrode attached to the positive terminal of a battery is the positive electrode, or anode., called a cathode close cathode the negative. The chloride ions are discharged (giving chlorine) in preference to the hydroxide ions. The one in the tin solution is the anode. The pt electrode in the permanganate solution is the cathode; The cathode (electrode in beaker. Positive Electrode Color.
From www.ezmedlearning.com
12 Lead ECG Placement Diagram and Mnemonic for Limb and Precordial Positive Electrode Color The chloride ions are discharged (giving chlorine) in preference to the hydroxide ions. The negative ions are attracted to the positive electrode. In a galvanic (voltaic) cell, the anode is considered negative and the cathode is considered positive. The redox reaction is not spontaneous and electrical energy has to be supplied to initiate the reaction. Chloride ions produce gaseous chlorine. Positive Electrode Color.
From www.cablesandsensors.com
12Lead ECG Placement The Ultimate Guide Positive Electrode Color This seems reasonable as the anode is the source of. A small spirit burner provides sufficient heat to melt the lead bromide. The positive electrode is a carbon fibre rod and the negative electrode is an iron nail. The negative ions are attracted to the positive electrode. The pt electrode in the permanganate solution is the cathode; Here, the redox. Positive Electrode Color.
From ecgwaves.com
The ECG leads electrodes, limb leads, chest (precordial) leads, 12 Positive Electrode Color Chloride ions produce gaseous chlorine bromide and iodide ions form bromine and iodine respectively which dissolve to form. The redox reaction is not spontaneous and electrical energy has to be supplied to initiate the reaction. A small spirit burner provides sufficient heat to melt the lead bromide. An electrolytic cell converts electrical energy into chemical energy. This seems reasonable as. Positive Electrode Color.
From www.researchgate.net
(PDF) Theoretical picture of positive electrode / solid electrolyte Positive Electrode Color A small spirit burner provides sufficient heat to melt the lead bromide. Here, the redox reaction is spontaneous and is responsible for the production of electrical energy. The chloride ions are discharged (giving chlorine) in preference to the hydroxide ions. The cathode (electrode in beaker that. This seems reasonable as the anode is the source of. The one in the. Positive Electrode Color.
From www.fdk.com
Positive electrode material │ FDK's original technology │ R&D │ FDK Positive Electrode Color The chloride ions are discharged (giving chlorine) in preference to the hydroxide ions. Chloride ions produce gaseous chlorine bromide and iodide ions form bromine and iodine respectively which dissolve to form. An electrolytic cell converts electrical energy into chemical energy. In a galvanic (voltaic) cell, the anode is considered negative and the cathode is considered positive. The negative ions are. Positive Electrode Color.
From mavink.com
Tungsten Color Code Chart Positive Electrode Color Here, the redox reaction is spontaneous and is responsible for the production of electrical energy. The chloride ions are discharged (giving chlorine) in preference to the hydroxide ions. In a galvanic (voltaic) cell, the anode is considered negative and the cathode is considered positive. The positive electrode is a carbon fibre rod and the negative electrode is an iron nail.. Positive Electrode Color.
From mungfali.com
Types Of Electrodes Positive Electrode Color The one in the tin solution is the anode. Chloride ions produce gaseous chlorine bromide and iodide ions form bromine and iodine respectively which dissolve to form. The redox reaction is not spontaneous and electrical energy has to be supplied to initiate the reaction. A small spirit burner provides sufficient heat to melt the lead bromide. An electrolytic cell converts. Positive Electrode Color.
From www.nagwa.com
Question Video Recalling the Name of the Positive Electrode in an Positive Electrode Color A small spirit burner provides sufficient heat to melt the lead bromide. This seems reasonable as the anode is the source of. Here, the redox reaction is spontaneous and is responsible for the production of electrical energy. The negative ions are attracted to the positive electrode. An electrolytic cell converts electrical energy into chemical energy. The electrode attached to the. Positive Electrode Color.
From www.worldatlas.com
What is the Difference Between a Cation and an Anion? WorldAtlas Positive Electrode Color Chloride ions produce gaseous chlorine bromide and iodide ions form bromine and iodine respectively which dissolve to form. The pt electrode in the permanganate solution is the cathode; This seems reasonable as the anode is the source of. The positive electrode is a carbon fibre rod and the negative electrode is an iron nail. The redox reaction is not spontaneous. Positive Electrode Color.
From www.fdk.com
Positive electrode material │ FDK's original technology │ R&D │ FDK Positive Electrode Color The electrode attached to the positive terminal of a battery is the positive electrode, or anode., called a cathode close cathode the negative. The positive electrode is a carbon fibre rod and the negative electrode is an iron nail. In a galvanic (voltaic) cell, the anode is considered negative and the cathode is considered positive. A small spirit burner provides. Positive Electrode Color.
From www.researchgate.net
Negative and positive electrode V(Q) curves vs Li/Li + before and after Positive Electrode Color A small spirit burner provides sufficient heat to melt the lead bromide. The negative ions are attracted to the positive electrode. The electrode attached to the positive terminal of a battery is the positive electrode, or anode., called a cathode close cathode the negative. An electrolytic cell converts electrical energy into chemical energy. The chloride ions are discharged (giving chlorine). Positive Electrode Color.
From kemele.labbyag.es
Tig Electrode Color Chart Kemele Positive Electrode Color An electrolytic cell converts electrical energy into chemical energy. The one in the tin solution is the anode. This seems reasonable as the anode is the source of. In a galvanic (voltaic) cell, the anode is considered negative and the cathode is considered positive. The cathode (electrode in beaker that. The pt electrode in the permanganate solution is the cathode;. Positive Electrode Color.
From www.ezmedlearning.com
How to Place a 5 Lead ECG Acronym, Mnemonic, Diagram for Electrode Positive Electrode Color A small spirit burner provides sufficient heat to melt the lead bromide. The electrode attached to the positive terminal of a battery is the positive electrode, or anode., called a cathode close cathode the negative. An electrolytic cell converts electrical energy into chemical energy. Here, the redox reaction is spontaneous and is responsible for the production of electrical energy. In. Positive Electrode Color.
From bastatrain.weebly.com
Cathode led positive or negative bastatrain Positive Electrode Color In a galvanic (voltaic) cell, the anode is considered negative and the cathode is considered positive. The pt electrode in the permanganate solution is the cathode; A small spirit burner provides sufficient heat to melt the lead bromide. An electrolytic cell converts electrical energy into chemical energy. Here, the redox reaction is spontaneous and is responsible for the production of. Positive Electrode Color.
From litfl.com
ECG Lead positioning • LITFL • ECG Library Basics Positive Electrode Color Here, the redox reaction is spontaneous and is responsible for the production of electrical energy. In a galvanic (voltaic) cell, the anode is considered negative and the cathode is considered positive. This seems reasonable as the anode is the source of. An electrolytic cell converts electrical energy into chemical energy. The positive electrode is a carbon fibre rod and the. Positive Electrode Color.
From www.researchgate.net
Images of the positive electrode (left), separator (middle) and the Positive Electrode Color A small spirit burner provides sufficient heat to melt the lead bromide. The cathode (electrode in beaker that. The negative ions are attracted to the positive electrode. Chloride ions produce gaseous chlorine bromide and iodide ions form bromine and iodine respectively which dissolve to form. The electrode attached to the positive terminal of a battery is the positive electrode, or. Positive Electrode Color.
From www.alamy.com
Diagram of the electrolysis of copper (II) chromate (VI) showing the Positive Electrode Color The chloride ions are discharged (giving chlorine) in preference to the hydroxide ions. The pt electrode in the permanganate solution is the cathode; This seems reasonable as the anode is the source of. The electrode attached to the positive terminal of a battery is the positive electrode, or anode., called a cathode close cathode the negative. The cathode (electrode in. Positive Electrode Color.
From ecg-educator.blogspot.com
ECG Educator Blog Introduction to the 12lead ECG Positive Electrode Color The one in the tin solution is the anode. A small spirit burner provides sufficient heat to melt the lead bromide. The pt electrode in the permanganate solution is the cathode; In a galvanic (voltaic) cell, the anode is considered negative and the cathode is considered positive. An electrolytic cell converts electrical energy into chemical energy. This seems reasonable as. Positive Electrode Color.
From edu.svet.gob.gt
TIG Tungsten Electrodes Explained (with Color Chart) Positive Electrode Color A small spirit burner provides sufficient heat to melt the lead bromide. The cathode (electrode in beaker that. The one in the tin solution is the anode. Here, the redox reaction is spontaneous and is responsible for the production of electrical energy. The redox reaction is not spontaneous and electrical energy has to be supplied to initiate the reaction. An. Positive Electrode Color.
From byjus.com
What is an active electrode? Explain with the help of an example Positive Electrode Color This seems reasonable as the anode is the source of. The positive electrode is a carbon fibre rod and the negative electrode is an iron nail. The chloride ions are discharged (giving chlorine) in preference to the hydroxide ions. The redox reaction is not spontaneous and electrical energy has to be supplied to initiate the reaction. Chloride ions produce gaseous. Positive Electrode Color.
From forum.facmedicine.com
Understanding ECGs Faculty of Medicine Positive Electrode Color The pt electrode in the permanganate solution is the cathode; A small spirit burner provides sufficient heat to melt the lead bromide. The cathode (electrode in beaker that. The redox reaction is not spontaneous and electrical energy has to be supplied to initiate the reaction. In a galvanic (voltaic) cell, the anode is considered negative and the cathode is considered. Positive Electrode Color.
From www.ezmedlearning.com
12 Lead ECG Placement Diagram and Mnemonic for Limb and Precordial Positive Electrode Color The positive electrode is a carbon fibre rod and the negative electrode is an iron nail. The cathode (electrode in beaker that. Chloride ions produce gaseous chlorine bromide and iodide ions form bromine and iodine respectively which dissolve to form. The pt electrode in the permanganate solution is the cathode; An electrolytic cell converts electrical energy into chemical energy. The. Positive Electrode Color.
From www.pinterest.co.uk
ECG. Color coding standards for 12lead ECG AHA and IEC Color coding Positive Electrode Color Here, the redox reaction is spontaneous and is responsible for the production of electrical energy. The one in the tin solution is the anode. The cathode (electrode in beaker that. The negative ions are attracted to the positive electrode. An electrolytic cell converts electrical energy into chemical energy. The positive electrode is a carbon fibre rod and the negative electrode. Positive Electrode Color.
From colorxml.com
Tungsten Welding Electrode Color Chart Colorxml Positive Electrode Color The redox reaction is not spontaneous and electrical energy has to be supplied to initiate the reaction. The chloride ions are discharged (giving chlorine) in preference to the hydroxide ions. The one in the tin solution is the anode. The negative ions are attracted to the positive electrode. The cathode (electrode in beaker that. A small spirit burner provides sufficient. Positive Electrode Color.
From mungfali.com
Tungsten Electrode Color Chart Positive Electrode Color The pt electrode in the permanganate solution is the cathode; The one in the tin solution is the anode. The chloride ions are discharged (giving chlorine) in preference to the hydroxide ions. The negative ions are attracted to the positive electrode. An electrolytic cell converts electrical energy into chemical energy. In a galvanic (voltaic) cell, the anode is considered negative. Positive Electrode Color.
From www.eromman.com
Buy Welding Electrode Pure Tungsten Colors (Green) eRomman Positive Electrode Color The chloride ions are discharged (giving chlorine) in preference to the hydroxide ions. The pt electrode in the permanganate solution is the cathode; The negative ions are attracted to the positive electrode. The electrode attached to the positive terminal of a battery is the positive electrode, or anode., called a cathode close cathode the negative. Here, the redox reaction is. Positive Electrode Color.
From www.slideserve.com
PPT Electrophoresis PowerPoint Presentation, free download ID175241 Positive Electrode Color Chloride ions produce gaseous chlorine bromide and iodide ions form bromine and iodine respectively which dissolve to form. The redox reaction is not spontaneous and electrical energy has to be supplied to initiate the reaction. This seems reasonable as the anode is the source of. The pt electrode in the permanganate solution is the cathode; In a galvanic (voltaic) cell,. Positive Electrode Color.