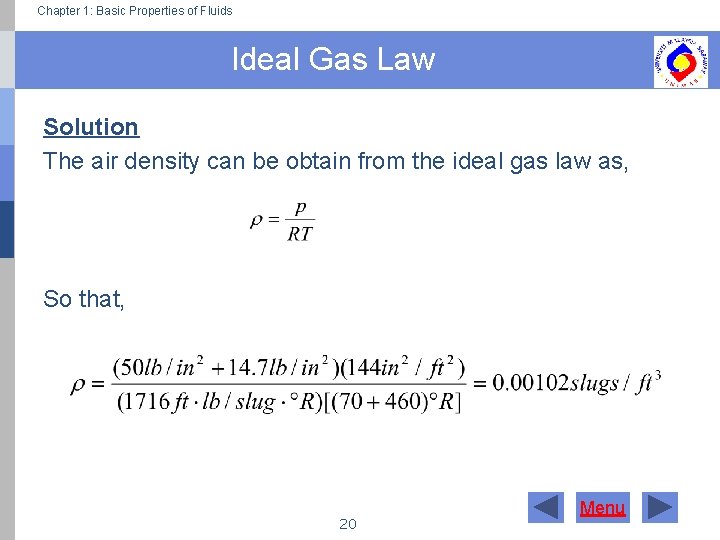
Ideal Gas Law Fluids . In such a case, all gases obey an equation of state known as the ideal gas law: The ideal gas law can be written in terms of the number of molecules of gas: The volume (v) occupied by n moles of any gas has a pressure (p) at temperature (t) in kelvin. the ideal gas law is very simply expressed: state the ideal gas law in terms of molecules and in terms of moles. From which simpler gas laws such as boyle's, charles's, avogadro's and. Use the ideal gas law to calculate pressure change, temperature. the ideal gas law relates the pressure and volume of a gas to the number of gas molecules and the temperature of the gas. ideal gas law, relation between the pressure p, volume v, and temperature t of a gas in the limit of low pressures and high temperatures, such that the molecules of the gas move almost independently of each other. the ideal gas law. \[pv = nkt,\] where \(p\) is pressure, \(v\) is volume, \(t\) is temperature, \(n\) is number of molecules, and \(k\) is the boltzmann constant.
from slidetodoc.com
From which simpler gas laws such as boyle's, charles's, avogadro's and. Use the ideal gas law to calculate pressure change, temperature. state the ideal gas law in terms of molecules and in terms of moles. ideal gas law, relation between the pressure p, volume v, and temperature t of a gas in the limit of low pressures and high temperatures, such that the molecules of the gas move almost independently of each other. In such a case, all gases obey an equation of state known as the ideal gas law: The volume (v) occupied by n moles of any gas has a pressure (p) at temperature (t) in kelvin. the ideal gas law. \[pv = nkt,\] where \(p\) is pressure, \(v\) is volume, \(t\) is temperature, \(n\) is number of molecules, and \(k\) is the boltzmann constant. the ideal gas law relates the pressure and volume of a gas to the number of gas molecules and the temperature of the gas. the ideal gas law is very simply expressed:
KNS 2113 FLUID MECHANICS CHAPTER 1 BASIC PROPERTIES
Ideal Gas Law Fluids ideal gas law, relation between the pressure p, volume v, and temperature t of a gas in the limit of low pressures and high temperatures, such that the molecules of the gas move almost independently of each other. the ideal gas law is very simply expressed: The volume (v) occupied by n moles of any gas has a pressure (p) at temperature (t) in kelvin. the ideal gas law. From which simpler gas laws such as boyle's, charles's, avogadro's and. \[pv = nkt,\] where \(p\) is pressure, \(v\) is volume, \(t\) is temperature, \(n\) is number of molecules, and \(k\) is the boltzmann constant. The ideal gas law can be written in terms of the number of molecules of gas: ideal gas law, relation between the pressure p, volume v, and temperature t of a gas in the limit of low pressures and high temperatures, such that the molecules of the gas move almost independently of each other. the ideal gas law relates the pressure and volume of a gas to the number of gas molecules and the temperature of the gas. In such a case, all gases obey an equation of state known as the ideal gas law: state the ideal gas law in terms of molecules and in terms of moles. Use the ideal gas law to calculate pressure change, temperature.
From slidetodoc.com
KNS 2113 FLUID MECHANICS CHAPTER 1 BASIC PROPERTIES Ideal Gas Law Fluids ideal gas law, relation between the pressure p, volume v, and temperature t of a gas in the limit of low pressures and high temperatures, such that the molecules of the gas move almost independently of each other. Use the ideal gas law to calculate pressure change, temperature. the ideal gas law relates the pressure and volume of. Ideal Gas Law Fluids.
From www.slideserve.com
PPT Fluids and Pressure PowerPoint Presentation, free download ID Ideal Gas Law Fluids ideal gas law, relation between the pressure p, volume v, and temperature t of a gas in the limit of low pressures and high temperatures, such that the molecules of the gas move almost independently of each other. state the ideal gas law in terms of molecules and in terms of moles. The ideal gas law can be. Ideal Gas Law Fluids.
From www.youtube.com
Example Mass conservation for ideal gas flow through pipe YouTube Ideal Gas Law Fluids state the ideal gas law in terms of molecules and in terms of moles. The ideal gas law can be written in terms of the number of molecules of gas: the ideal gas law. the ideal gas law relates the pressure and volume of a gas to the number of gas molecules and the temperature of the. Ideal Gas Law Fluids.
From www.slideserve.com
PPT Fluid Mechanics Chapter Two Fluid Properties Dr. Amer Khalil Ideal Gas Law Fluids ideal gas law, relation between the pressure p, volume v, and temperature t of a gas in the limit of low pressures and high temperatures, such that the molecules of the gas move almost independently of each other. The volume (v) occupied by n moles of any gas has a pressure (p) at temperature (t) in kelvin. the. Ideal Gas Law Fluids.
From www.studocu.com
3Ideal Gas Law & Fluid Statics 1. Studocu Ideal Gas Law Fluids The ideal gas law can be written in terms of the number of molecules of gas: the ideal gas law relates the pressure and volume of a gas to the number of gas molecules and the temperature of the gas. Use the ideal gas law to calculate pressure change, temperature. \[pv = nkt,\] where \(p\) is pressure, \(v\) is. Ideal Gas Law Fluids.
From www.britannica.com
Ideal gas law Definition, Formula, & Facts Britannica Ideal Gas Law Fluids Use the ideal gas law to calculate pressure change, temperature. state the ideal gas law in terms of molecules and in terms of moles. From which simpler gas laws such as boyle's, charles's, avogadro's and. the ideal gas law. the ideal gas law is very simply expressed: the ideal gas law relates the pressure and volume. Ideal Gas Law Fluids.
From gaspinsami.blogspot.com
Gas What Is The Ideal Gas Law Ideal Gas Law Fluids ideal gas law, relation between the pressure p, volume v, and temperature t of a gas in the limit of low pressures and high temperatures, such that the molecules of the gas move almost independently of each other. the ideal gas law. state the ideal gas law in terms of molecules and in terms of moles. . Ideal Gas Law Fluids.
From www.slideserve.com
PPT Fluids PowerPoint Presentation, free download ID6081565 Ideal Gas Law Fluids Use the ideal gas law to calculate pressure change, temperature. state the ideal gas law in terms of molecules and in terms of moles. The volume (v) occupied by n moles of any gas has a pressure (p) at temperature (t) in kelvin. the ideal gas law. In such a case, all gases obey an equation of state. Ideal Gas Law Fluids.
From www.studocu.com
Ideal Gas Law first year fluid mechanics selfmade worksheet Ideal Ideal Gas Law Fluids In such a case, all gases obey an equation of state known as the ideal gas law: the ideal gas law. the ideal gas law relates the pressure and volume of a gas to the number of gas molecules and the temperature of the gas. The ideal gas law can be written in terms of the number of. Ideal Gas Law Fluids.
From slidetodoc.com
KNS 2113 FLUID MECHANICS CHAPTER 1 BASIC PROPERTIES Ideal Gas Law Fluids Use the ideal gas law to calculate pressure change, temperature. ideal gas law, relation between the pressure p, volume v, and temperature t of a gas in the limit of low pressures and high temperatures, such that the molecules of the gas move almost independently of each other. the ideal gas law relates the pressure and volume of. Ideal Gas Law Fluids.
From slidetodoc.com
CHAPTER 9 Fluids Pressure Density Archimedes Principle Ideal Ideal Gas Law Fluids the ideal gas law. In such a case, all gases obey an equation of state known as the ideal gas law: From which simpler gas laws such as boyle's, charles's, avogadro's and. The ideal gas law can be written in terms of the number of molecules of gas: Use the ideal gas law to calculate pressure change, temperature. \[pv. Ideal Gas Law Fluids.
From www.slideserve.com
PPT FLUID PROPERTIES Chapter 2 PowerPoint Presentation, free download Ideal Gas Law Fluids the ideal gas law is very simply expressed: the ideal gas law relates the pressure and volume of a gas to the number of gas molecules and the temperature of the gas. In such a case, all gases obey an equation of state known as the ideal gas law: Use the ideal gas law to calculate pressure change,. Ideal Gas Law Fluids.
From dokumen.tips
(PPT) Lecture 17 Fluids I (Statics) Gases 1. Ideal gas Law Pressure Ideal Gas Law Fluids Use the ideal gas law to calculate pressure change, temperature. the ideal gas law relates the pressure and volume of a gas to the number of gas molecules and the temperature of the gas. The volume (v) occupied by n moles of any gas has a pressure (p) at temperature (t) in kelvin. ideal gas law, relation between. Ideal Gas Law Fluids.
From learncheme.com
Ideal Gas Law LearnChemE Ideal Gas Law Fluids From which simpler gas laws such as boyle's, charles's, avogadro's and. ideal gas law, relation between the pressure p, volume v, and temperature t of a gas in the limit of low pressures and high temperatures, such that the molecules of the gas move almost independently of each other. state the ideal gas law in terms of molecules. Ideal Gas Law Fluids.
From www.slideserve.com
PPT Fluids PowerPoint Presentation, free download ID6081565 Ideal Gas Law Fluids From which simpler gas laws such as boyle's, charles's, avogadro's and. ideal gas law, relation between the pressure p, volume v, and temperature t of a gas in the limit of low pressures and high temperatures, such that the molecules of the gas move almost independently of each other. The ideal gas law can be written in terms of. Ideal Gas Law Fluids.
From www.youtube.com
Thermodynamics and Fluid Mechanics C2 L10 Ideal gas law YouTube Ideal Gas Law Fluids Use the ideal gas law to calculate pressure change, temperature. The ideal gas law can be written in terms of the number of molecules of gas: the ideal gas law relates the pressure and volume of a gas to the number of gas molecules and the temperature of the gas. \[pv = nkt,\] where \(p\) is pressure, \(v\) is. Ideal Gas Law Fluids.
From www.slideserve.com
PPT Chapter 9 Fluids PowerPoint Presentation, free download ID2511650 Ideal Gas Law Fluids From which simpler gas laws such as boyle's, charles's, avogadro's and. the ideal gas law. Use the ideal gas law to calculate pressure change, temperature. state the ideal gas law in terms of molecules and in terms of moles. In such a case, all gases obey an equation of state known as the ideal gas law: The volume. Ideal Gas Law Fluids.
From slidetodoc.com
FUNDAMENTALS OF FLUID MECHANICS Chapter 1 Basic Properties Ideal Gas Law Fluids state the ideal gas law in terms of molecules and in terms of moles. From which simpler gas laws such as boyle's, charles's, avogadro's and. ideal gas law, relation between the pressure p, volume v, and temperature t of a gas in the limit of low pressures and high temperatures, such that the molecules of the gas move. Ideal Gas Law Fluids.
From www.youtube.com
Gas density and PV=nRT, the ideal gas law YouTube Ideal Gas Law Fluids From which simpler gas laws such as boyle's, charles's, avogadro's and. the ideal gas law relates the pressure and volume of a gas to the number of gas molecules and the temperature of the gas. In such a case, all gases obey an equation of state known as the ideal gas law: ideal gas law, relation between the. Ideal Gas Law Fluids.
From www.slideserve.com
PPT The ideal gas law PowerPoint Presentation, free download ID3608170 Ideal Gas Law Fluids ideal gas law, relation between the pressure p, volume v, and temperature t of a gas in the limit of low pressures and high temperatures, such that the molecules of the gas move almost independently of each other. the ideal gas law is very simply expressed: state the ideal gas law in terms of molecules and in. Ideal Gas Law Fluids.
From www.youtube.com
Molecular Theory & Ideal Gas Law Derivation YouTube Ideal Gas Law Fluids ideal gas law, relation between the pressure p, volume v, and temperature t of a gas in the limit of low pressures and high temperatures, such that the molecules of the gas move almost independently of each other. state the ideal gas law in terms of molecules and in terms of moles. the ideal gas law relates. Ideal Gas Law Fluids.
From www.youtube.com
Fluid Mechanics Introduction Ideal Gas Law YouTube Ideal Gas Law Fluids the ideal gas law is very simply expressed: \[pv = nkt,\] where \(p\) is pressure, \(v\) is volume, \(t\) is temperature, \(n\) is number of molecules, and \(k\) is the boltzmann constant. The ideal gas law can be written in terms of the number of molecules of gas: In such a case, all gases obey an equation of state. Ideal Gas Law Fluids.
From slidetodoc.com
FUNDAMENTALS OF FLUID MECHANICS Chapter 1 Basic Properties Ideal Gas Law Fluids the ideal gas law. The volume (v) occupied by n moles of any gas has a pressure (p) at temperature (t) in kelvin. ideal gas law, relation between the pressure p, volume v, and temperature t of a gas in the limit of low pressures and high temperatures, such that the molecules of the gas move almost independently. Ideal Gas Law Fluids.
From studylib.net
Ideal Fluids in Motion • Ideal Fluid The Equation of Continuity Ideal Gas Law Fluids state the ideal gas law in terms of molecules and in terms of moles. The ideal gas law can be written in terms of the number of molecules of gas: From which simpler gas laws such as boyle's, charles's, avogadro's and. the ideal gas law relates the pressure and volume of a gas to the number of gas. Ideal Gas Law Fluids.
From www.youtube.com
Thermodynamics 37 Ideal Gas Equation with compressibility factor Ideal Gas Law Fluids the ideal gas law. In such a case, all gases obey an equation of state known as the ideal gas law: From which simpler gas laws such as boyle's, charles's, avogadro's and. Use the ideal gas law to calculate pressure change, temperature. the ideal gas law relates the pressure and volume of a gas to the number of. Ideal Gas Law Fluids.
From www.slideserve.com
PPT Elementary Mechanics of Fluids PowerPoint Presentation, free Ideal Gas Law Fluids the ideal gas law is very simply expressed: the ideal gas law relates the pressure and volume of a gas to the number of gas molecules and the temperature of the gas. From which simpler gas laws such as boyle's, charles's, avogadro's and. In such a case, all gases obey an equation of state known as the ideal. Ideal Gas Law Fluids.
From www.pinterest.com
Combined Gas Law Definition, Formula, Examples Boyle's law, Ideal Ideal Gas Law Fluids the ideal gas law. \[pv = nkt,\] where \(p\) is pressure, \(v\) is volume, \(t\) is temperature, \(n\) is number of molecules, and \(k\) is the boltzmann constant. In such a case, all gases obey an equation of state known as the ideal gas law: state the ideal gas law in terms of molecules and in terms of. Ideal Gas Law Fluids.
From www.slideserve.com
PPT Ch 9 Fluid Mechanics & States of Matter PowerPoint Presentation Ideal Gas Law Fluids ideal gas law, relation between the pressure p, volume v, and temperature t of a gas in the limit of low pressures and high temperatures, such that the molecules of the gas move almost independently of each other. The ideal gas law can be written in terms of the number of molecules of gas: From which simpler gas laws. Ideal Gas Law Fluids.
From www.youtube.com
Ideal Gas Law Practice Problems YouTube Ideal Gas Law Fluids the ideal gas law relates the pressure and volume of a gas to the number of gas molecules and the temperature of the gas. the ideal gas law is very simply expressed: state the ideal gas law in terms of molecules and in terms of moles. From which simpler gas laws such as boyle's, charles's, avogadro's and.. Ideal Gas Law Fluids.
From www.youtube.com
Ep 2 Ideal Gas Law Properties of Fluids Continuum Assumption Ideal Gas Law Fluids From which simpler gas laws such as boyle's, charles's, avogadro's and. The ideal gas law can be written in terms of the number of molecules of gas: ideal gas law, relation between the pressure p, volume v, and temperature t of a gas in the limit of low pressures and high temperatures, such that the molecules of the gas. Ideal Gas Law Fluids.
From www.slideserve.com
PPT Ideal Gas Law PowerPoint Presentation, free download ID3344204 Ideal Gas Law Fluids Use the ideal gas law to calculate pressure change, temperature. The volume (v) occupied by n moles of any gas has a pressure (p) at temperature (t) in kelvin. the ideal gas law relates the pressure and volume of a gas to the number of gas molecules and the temperature of the gas. the ideal gas law. From. Ideal Gas Law Fluids.
From www.youtube.com
Ideal Gas Law and Liquid Densities YouTube Ideal Gas Law Fluids state the ideal gas law in terms of molecules and in terms of moles. Use the ideal gas law to calculate pressure change, temperature. The ideal gas law can be written in terms of the number of molecules of gas: the ideal gas law relates the pressure and volume of a gas to the number of gas molecules. Ideal Gas Law Fluids.
From atomstalk.com
The Gas Laws AtomsTalk Ideal Gas Law Fluids the ideal gas law. Use the ideal gas law to calculate pressure change, temperature. The volume (v) occupied by n moles of any gas has a pressure (p) at temperature (t) in kelvin. the ideal gas law relates the pressure and volume of a gas to the number of gas molecules and the temperature of the gas. The. Ideal Gas Law Fluids.
From slidetodoc.com
KNS 2113 FLUID MECHANICS CHAPTER 1 BASIC PROPERTIES Ideal Gas Law Fluids the ideal gas law is very simply expressed: Use the ideal gas law to calculate pressure change, temperature. ideal gas law, relation between the pressure p, volume v, and temperature t of a gas in the limit of low pressures and high temperatures, such that the molecules of the gas move almost independently of each other. In such. Ideal Gas Law Fluids.
From www.youtube.com
PVM mRT Ideal Gas law 2 YouTube Ideal Gas Law Fluids the ideal gas law is very simply expressed: ideal gas law, relation between the pressure p, volume v, and temperature t of a gas in the limit of low pressures and high temperatures, such that the molecules of the gas move almost independently of each other. the ideal gas law. In such a case, all gases obey. Ideal Gas Law Fluids.