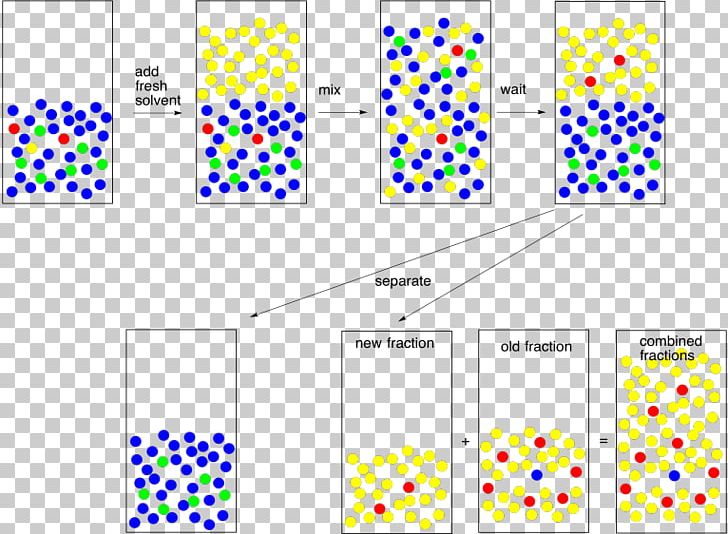
Liquid Extraction Polarity . The other solvent is a liquid that does not dissolve very well in water, such as diethyl ether (this is the most common type of ether, and it is often called simply ether). The liquids involved have to be immiscible in. for more polar analytes, and here lower values of logp(d) can also act as a good indicator of polarity, one may need to. Usually one of the solvents is water. solvent partitioning requires two solvents that are not miscible in each other.
from imgbin.com
The other solvent is a liquid that does not dissolve very well in water, such as diethyl ether (this is the most common type of ether, and it is often called simply ether). for more polar analytes, and here lower values of logp(d) can also act as a good indicator of polarity, one may need to. Usually one of the solvents is water. solvent partitioning requires two solvents that are not miscible in each other. The liquids involved have to be immiscible in.
Ether Liquidliquid Extraction Solvent In Chemical Reactions Chemical
Liquid Extraction Polarity Usually one of the solvents is water. The other solvent is a liquid that does not dissolve very well in water, such as diethyl ether (this is the most common type of ether, and it is often called simply ether). The liquids involved have to be immiscible in. Usually one of the solvents is water. solvent partitioning requires two solvents that are not miscible in each other. for more polar analytes, and here lower values of logp(d) can also act as a good indicator of polarity, one may need to.
From ecolink.com
The Process of LiquidLiquid Extraction EcoLink Inc. Liquid Extraction Polarity for more polar analytes, and here lower values of logp(d) can also act as a good indicator of polarity, one may need to. solvent partitioning requires two solvents that are not miscible in each other. The liquids involved have to be immiscible in. Usually one of the solvents is water. The other solvent is a liquid that does. Liquid Extraction Polarity.
From www.studypool.com
SOLUTION Physical chemistry liquid liquid extraction Studypool Liquid Extraction Polarity for more polar analytes, and here lower values of logp(d) can also act as a good indicator of polarity, one may need to. The other solvent is a liquid that does not dissolve very well in water, such as diethyl ether (this is the most common type of ether, and it is often called simply ether). Usually one of. Liquid Extraction Polarity.
From www.slideserve.com
PPT Chapter 12 SOLUTIONS PowerPoint Presentation, free download ID Liquid Extraction Polarity The other solvent is a liquid that does not dissolve very well in water, such as diethyl ether (this is the most common type of ether, and it is often called simply ether). solvent partitioning requires two solvents that are not miscible in each other. The liquids involved have to be immiscible in. for more polar analytes, and. Liquid Extraction Polarity.
From www.chegg.com
LiquidLiquid Extraction Cascade System Analysis The Liquid Extraction Polarity Usually one of the solvents is water. solvent partitioning requires two solvents that are not miscible in each other. The liquids involved have to be immiscible in. for more polar analytes, and here lower values of logp(d) can also act as a good indicator of polarity, one may need to. The other solvent is a liquid that does. Liquid Extraction Polarity.
From www.researchgate.net
Schematic of liquidliquid extraction process. The organic phase is Liquid Extraction Polarity for more polar analytes, and here lower values of logp(d) can also act as a good indicator of polarity, one may need to. The liquids involved have to be immiscible in. The other solvent is a liquid that does not dissolve very well in water, such as diethyl ether (this is the most common type of ether, and it. Liquid Extraction Polarity.
From www.researchgate.net
Polarity of Solvents Used for Extraction Download Table Liquid Extraction Polarity The liquids involved have to be immiscible in. for more polar analytes, and here lower values of logp(d) can also act as a good indicator of polarity, one may need to. solvent partitioning requires two solvents that are not miscible in each other. The other solvent is a liquid that does not dissolve very well in water, such. Liquid Extraction Polarity.
From www.researchgate.net
4 Schematic representation of pressurized liquid extraction (PLE Liquid Extraction Polarity Usually one of the solvents is water. The liquids involved have to be immiscible in. The other solvent is a liquid that does not dissolve very well in water, such as diethyl ether (this is the most common type of ether, and it is often called simply ether). solvent partitioning requires two solvents that are not miscible in each. Liquid Extraction Polarity.
From www.researchgate.net
5 Different configurations for liquidliquid extraction of compounds Liquid Extraction Polarity Usually one of the solvents is water. solvent partitioning requires two solvents that are not miscible in each other. The liquids involved have to be immiscible in. The other solvent is a liquid that does not dissolve very well in water, such as diethyl ether (this is the most common type of ether, and it is often called simply. Liquid Extraction Polarity.
From www.researchgate.net
Liquidliquid extractions involving (a) conventional organic solvents Liquid Extraction Polarity for more polar analytes, and here lower values of logp(d) can also act as a good indicator of polarity, one may need to. The other solvent is a liquid that does not dissolve very well in water, such as diethyl ether (this is the most common type of ether, and it is often called simply ether). The liquids involved. Liquid Extraction Polarity.
From www.researchgate.net
Schematic of the organic gas steamliquid extraction setup Download Liquid Extraction Polarity The liquids involved have to be immiscible in. The other solvent is a liquid that does not dissolve very well in water, such as diethyl ether (this is the most common type of ether, and it is often called simply ether). solvent partitioning requires two solvents that are not miscible in each other. for more polar analytes, and. Liquid Extraction Polarity.
From chem.libretexts.org
4.2 Overview of Extraction Chemistry LibreTexts Liquid Extraction Polarity Usually one of the solvents is water. solvent partitioning requires two solvents that are not miscible in each other. for more polar analytes, and here lower values of logp(d) can also act as a good indicator of polarity, one may need to. The other solvent is a liquid that does not dissolve very well in water, such as. Liquid Extraction Polarity.
From imgbin.com
Ether Liquidliquid Extraction Solvent In Chemical Reactions Chemical Liquid Extraction Polarity Usually one of the solvents is water. for more polar analytes, and here lower values of logp(d) can also act as a good indicator of polarity, one may need to. solvent partitioning requires two solvents that are not miscible in each other. The other solvent is a liquid that does not dissolve very well in water, such as. Liquid Extraction Polarity.
From eduinput.com
Solvent Extraction Types, Principle, uses Liquid Extraction Polarity The other solvent is a liquid that does not dissolve very well in water, such as diethyl ether (this is the most common type of ether, and it is often called simply ether). solvent partitioning requires two solvents that are not miscible in each other. The liquids involved have to be immiscible in. Usually one of the solvents is. Liquid Extraction Polarity.
From chem.libretexts.org
7.7 LiquidLiquid Extractions Chemistry LibreTexts Liquid Extraction Polarity Usually one of the solvents is water. for more polar analytes, and here lower values of logp(d) can also act as a good indicator of polarity, one may need to. The liquids involved have to be immiscible in. The other solvent is a liquid that does not dissolve very well in water, such as diethyl ether (this is the. Liquid Extraction Polarity.
From mungfali.com
Solvent Polarity Chart Liquid Extraction Polarity The liquids involved have to be immiscible in. The other solvent is a liquid that does not dissolve very well in water, such as diethyl ether (this is the most common type of ether, and it is often called simply ether). for more polar analytes, and here lower values of logp(d) can also act as a good indicator of. Liquid Extraction Polarity.
From www.researchgate.net
Schematic representation of the solidliquid extraction technique for Liquid Extraction Polarity solvent partitioning requires two solvents that are not miscible in each other. for more polar analytes, and here lower values of logp(d) can also act as a good indicator of polarity, one may need to. The liquids involved have to be immiscible in. Usually one of the solvents is water. The other solvent is a liquid that does. Liquid Extraction Polarity.
From www.researchgate.net
Polarity and physical properties of extraction solvents. Download Liquid Extraction Polarity Usually one of the solvents is water. The other solvent is a liquid that does not dissolve very well in water, such as diethyl ether (this is the most common type of ether, and it is often called simply ether). for more polar analytes, and here lower values of logp(d) can also act as a good indicator of polarity,. Liquid Extraction Polarity.
From chem.libretexts.org
7.7 LiquidLiquid Extractions Chemistry LibreTexts Liquid Extraction Polarity Usually one of the solvents is water. solvent partitioning requires two solvents that are not miscible in each other. for more polar analytes, and here lower values of logp(d) can also act as a good indicator of polarity, one may need to. The other solvent is a liquid that does not dissolve very well in water, such as. Liquid Extraction Polarity.
From www.slideserve.com
PPT Liquid Liquid Extraction PowerPoint Presentation, free download Liquid Extraction Polarity The other solvent is a liquid that does not dissolve very well in water, such as diethyl ether (this is the most common type of ether, and it is often called simply ether). solvent partitioning requires two solvents that are not miscible in each other. The liquids involved have to be immiscible in. for more polar analytes, and. Liquid Extraction Polarity.
From eduinput.com
Types of Solvent Extraction Solvent Extraction Techniques Liquid Extraction Polarity Usually one of the solvents is water. The other solvent is a liquid that does not dissolve very well in water, such as diethyl ether (this is the most common type of ether, and it is often called simply ether). solvent partitioning requires two solvents that are not miscible in each other. The liquids involved have to be immiscible. Liquid Extraction Polarity.
From bceweb.org
Solvent Polarity Chart A Visual Reference of Charts Chart Master Liquid Extraction Polarity The other solvent is a liquid that does not dissolve very well in water, such as diethyl ether (this is the most common type of ether, and it is often called simply ether). solvent partitioning requires two solvents that are not miscible in each other. for more polar analytes, and here lower values of logp(d) can also act. Liquid Extraction Polarity.
From www.slideserve.com
PPT Liquid Liquid Extraction PowerPoint Presentation, free download Liquid Extraction Polarity for more polar analytes, and here lower values of logp(d) can also act as a good indicator of polarity, one may need to. The other solvent is a liquid that does not dissolve very well in water, such as diethyl ether (this is the most common type of ether, and it is often called simply ether). The liquids involved. Liquid Extraction Polarity.
From www.researchgate.net
A diagrammatic representation of pressurized liquid extraction Liquid Extraction Polarity solvent partitioning requires two solvents that are not miscible in each other. Usually one of the solvents is water. for more polar analytes, and here lower values of logp(d) can also act as a good indicator of polarity, one may need to. The liquids involved have to be immiscible in. The other solvent is a liquid that does. Liquid Extraction Polarity.
From www.researchgate.net
Schematic illustration of the fabrication, gradient extraction, and Liquid Extraction Polarity Usually one of the solvents is water. The other solvent is a liquid that does not dissolve very well in water, such as diethyl ether (this is the most common type of ether, and it is often called simply ether). solvent partitioning requires two solvents that are not miscible in each other. The liquids involved have to be immiscible. Liquid Extraction Polarity.
From www.youtube.com
classification of extractionliquidliquid extractionsolid phase Liquid Extraction Polarity solvent partitioning requires two solvents that are not miscible in each other. for more polar analytes, and here lower values of logp(d) can also act as a good indicator of polarity, one may need to. The other solvent is a liquid that does not dissolve very well in water, such as diethyl ether (this is the most common. Liquid Extraction Polarity.
From www.researchgate.net
Flow chart of liquidsolid extraction in stages from jayanti (S. sesban Liquid Extraction Polarity Usually one of the solvents is water. The liquids involved have to be immiscible in. solvent partitioning requires two solvents that are not miscible in each other. The other solvent is a liquid that does not dissolve very well in water, such as diethyl ether (this is the most common type of ether, and it is often called simply. Liquid Extraction Polarity.
From www.slideserve.com
PPT Solutions PowerPoint Presentation, free download ID269800 Liquid Extraction Polarity Usually one of the solvents is water. solvent partitioning requires two solvents that are not miscible in each other. The other solvent is a liquid that does not dissolve very well in water, such as diethyl ether (this is the most common type of ether, and it is often called simply ether). for more polar analytes, and here. Liquid Extraction Polarity.
From www.chegg.com
Solved Determine the solvent polarity index for the Liquid Extraction Polarity The liquids involved have to be immiscible in. Usually one of the solvents is water. The other solvent is a liquid that does not dissolve very well in water, such as diethyl ether (this is the most common type of ether, and it is often called simply ether). solvent partitioning requires two solvents that are not miscible in each. Liquid Extraction Polarity.
From www.youtube.com
Liquid Liquid Extraction Process What is Solvent Extraction Process Liquid Extraction Polarity Usually one of the solvents is water. solvent partitioning requires two solvents that are not miscible in each other. for more polar analytes, and here lower values of logp(d) can also act as a good indicator of polarity, one may need to. The other solvent is a liquid that does not dissolve very well in water, such as. Liquid Extraction Polarity.
From pubs.acs.org
A Guided InquiryBased LiquidLiquid Extraction Liquid Extraction Polarity The other solvent is a liquid that does not dissolve very well in water, such as diethyl ether (this is the most common type of ether, and it is often called simply ether). solvent partitioning requires two solvents that are not miscible in each other. The liquids involved have to be immiscible in. Usually one of the solvents is. Liquid Extraction Polarity.
From www.researchgate.net
Diagrammatic steps of solid phase extraction (adapted from Badawy et Liquid Extraction Polarity The other solvent is a liquid that does not dissolve very well in water, such as diethyl ether (this is the most common type of ether, and it is often called simply ether). The liquids involved have to be immiscible in. solvent partitioning requires two solvents that are not miscible in each other. for more polar analytes, and. Liquid Extraction Polarity.
From www.researchgate.net
Polarity and physical properties of extraction solvents. Download Liquid Extraction Polarity solvent partitioning requires two solvents that are not miscible in each other. The other solvent is a liquid that does not dissolve very well in water, such as diethyl ether (this is the most common type of ether, and it is often called simply ether). The liquids involved have to be immiscible in. for more polar analytes, and. Liquid Extraction Polarity.
From www.slideserve.com
PPT Solubility and Extraction PowerPoint Presentation, free download Liquid Extraction Polarity The liquids involved have to be immiscible in. for more polar analytes, and here lower values of logp(d) can also act as a good indicator of polarity, one may need to. Usually one of the solvents is water. solvent partitioning requires two solvents that are not miscible in each other. The other solvent is a liquid that does. Liquid Extraction Polarity.
From techblog.ctgclean.com
Chemistry Solvent Characteristics CTG Clean Liquid Extraction Polarity The liquids involved have to be immiscible in. The other solvent is a liquid that does not dissolve very well in water, such as diethyl ether (this is the most common type of ether, and it is often called simply ether). solvent partitioning requires two solvents that are not miscible in each other. for more polar analytes, and. Liquid Extraction Polarity.
From www.coursehero.com
[Solved] draw rxn mechanism diagram. 5. In the liquidliquid extraction Liquid Extraction Polarity Usually one of the solvents is water. The other solvent is a liquid that does not dissolve very well in water, such as diethyl ether (this is the most common type of ether, and it is often called simply ether). for more polar analytes, and here lower values of logp(d) can also act as a good indicator of polarity,. Liquid Extraction Polarity.