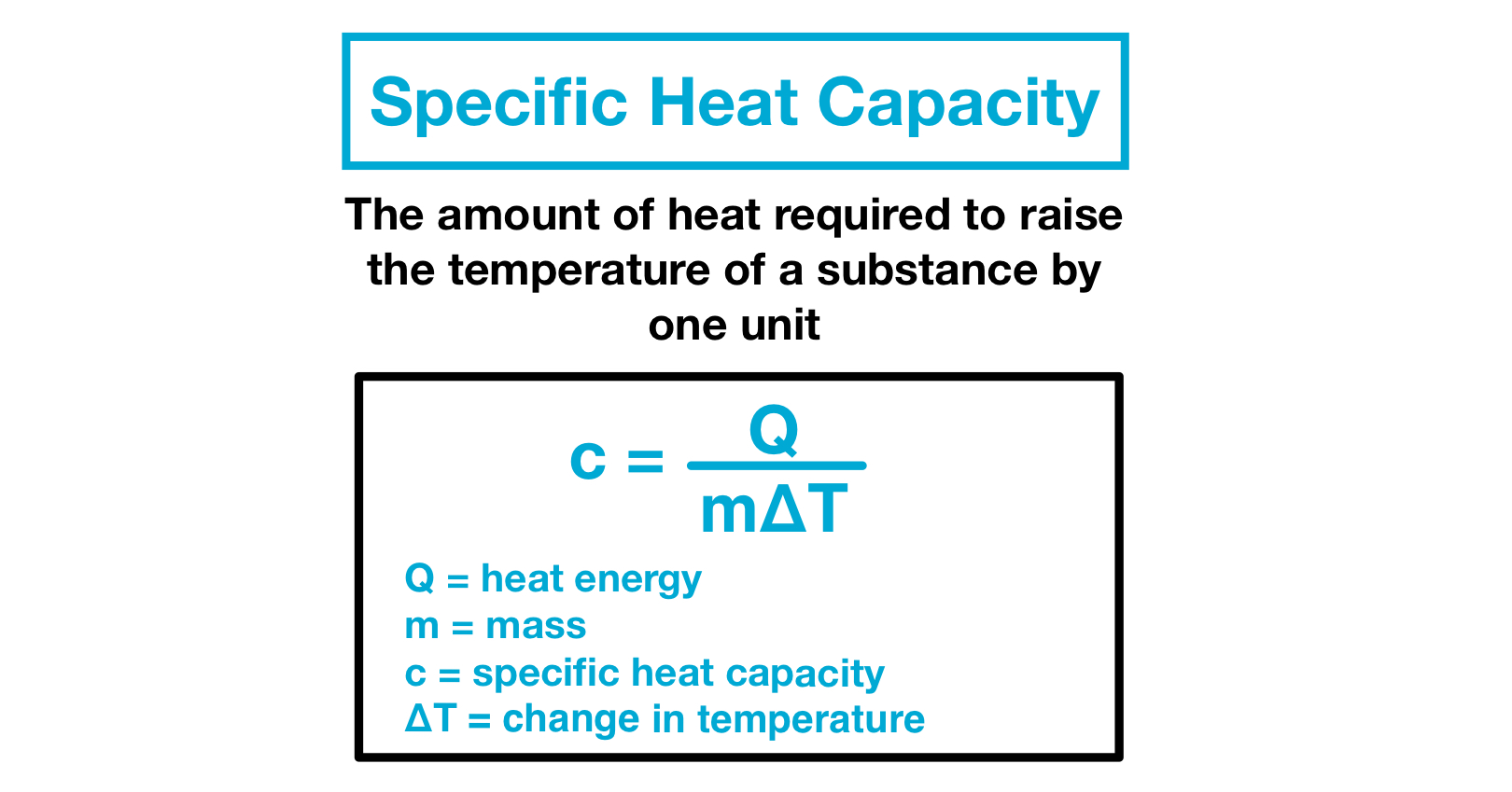
Heat Capacity In Calorimetry . the heat capacity of the calorimeter or of the reaction mixture may be used to calculate the amount of heat released or absorbed. one technique we can use to measure the amount of heat involved in a chemical or physical process is known as calorimetry. the calorimetry calculator can help you solve complex calorimetry problems. It can analyze the heat exchange between up to 3 objects. a calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. q = mcδt, where q is the symbol for heat transfer (“quantity of heat”), m is the mass of the substance, and δt is the change in temperature. when analysing a heat transfer reaction, chemists use the calorimetry equation relating heat released in the reaction to the substance’s mass, change in. The specific heat capacity (c) of a substance is the heating required to raise the temperature of 1 g of a. Calorimetry is used to measure.
from www.expii.com
when analysing a heat transfer reaction, chemists use the calorimetry equation relating heat released in the reaction to the substance’s mass, change in. Calorimetry is used to measure. a calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. one technique we can use to measure the amount of heat involved in a chemical or physical process is known as calorimetry. the heat capacity of the calorimeter or of the reaction mixture may be used to calculate the amount of heat released or absorbed. It can analyze the heat exchange between up to 3 objects. q = mcδt, where q is the symbol for heat transfer (“quantity of heat”), m is the mass of the substance, and δt is the change in temperature. The specific heat capacity (c) of a substance is the heating required to raise the temperature of 1 g of a. the calorimetry calculator can help you solve complex calorimetry problems.
Heat Capacity of Water — Overview & Importance Expii
Heat Capacity In Calorimetry q = mcδt, where q is the symbol for heat transfer (“quantity of heat”), m is the mass of the substance, and δt is the change in temperature. one technique we can use to measure the amount of heat involved in a chemical or physical process is known as calorimetry. q = mcδt, where q is the symbol for heat transfer (“quantity of heat”), m is the mass of the substance, and δt is the change in temperature. It can analyze the heat exchange between up to 3 objects. a calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. Calorimetry is used to measure. the heat capacity of the calorimeter or of the reaction mixture may be used to calculate the amount of heat released or absorbed. The specific heat capacity (c) of a substance is the heating required to raise the temperature of 1 g of a. when analysing a heat transfer reaction, chemists use the calorimetry equation relating heat released in the reaction to the substance’s mass, change in. the calorimetry calculator can help you solve complex calorimetry problems.
From grade12uchemistry.weebly.com
Calorimetry Grade12UChemistry Heat Capacity In Calorimetry one technique we can use to measure the amount of heat involved in a chemical or physical process is known as calorimetry. a calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. It can analyze the heat exchange between up to 3 objects. Calorimetry is used to measure. . Heat Capacity In Calorimetry.
From www.slideshare.net
Tang 01 heat capacity and calorimetry Heat Capacity In Calorimetry Calorimetry is used to measure. the heat capacity of the calorimeter or of the reaction mixture may be used to calculate the amount of heat released or absorbed. It can analyze the heat exchange between up to 3 objects. a calorimeter is a device used to measure the amount of heat involved in a chemical or physical process.. Heat Capacity In Calorimetry.
From www.youtube.com
Principle of Calorimetry YouTube Heat Capacity In Calorimetry q = mcδt, where q is the symbol for heat transfer (“quantity of heat”), m is the mass of the substance, and δt is the change in temperature. the heat capacity of the calorimeter or of the reaction mixture may be used to calculate the amount of heat released or absorbed. when analysing a heat transfer reaction,. Heat Capacity In Calorimetry.
From www.studocu.com
Heat Capacity Calorimetry Worksheet answers NAME_________________PER Heat Capacity In Calorimetry q = mcδt, where q is the symbol for heat transfer (“quantity of heat”), m is the mass of the substance, and δt is the change in temperature. the heat capacity of the calorimeter or of the reaction mixture may be used to calculate the amount of heat released or absorbed. The specific heat capacity (c) of a. Heat Capacity In Calorimetry.
From pressbooks.online.ucf.edu
10.2 Calorimetry Chemistry Fundamentals Heat Capacity In Calorimetry Calorimetry is used to measure. It can analyze the heat exchange between up to 3 objects. the calorimetry calculator can help you solve complex calorimetry problems. The specific heat capacity (c) of a substance is the heating required to raise the temperature of 1 g of a. when analysing a heat transfer reaction, chemists use the calorimetry equation. Heat Capacity In Calorimetry.
From www.youtube.com
Calorimetry Heat Capacity Example AP Chemistry YouTube Heat Capacity In Calorimetry a calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. one technique we can use to measure the amount of heat involved in a chemical or physical process is known as calorimetry. The specific heat capacity (c) of a substance is the heating required to raise the temperature of. Heat Capacity In Calorimetry.
From studymind.co.uk
Heat Capacity Study Mind Heat Capacity In Calorimetry the calorimetry calculator can help you solve complex calorimetry problems. the heat capacity of the calorimeter or of the reaction mixture may be used to calculate the amount of heat released or absorbed. when analysing a heat transfer reaction, chemists use the calorimetry equation relating heat released in the reaction to the substance’s mass, change in. It. Heat Capacity In Calorimetry.
From www.tec-science.com
Calorimeter to determine the specific heat capacities of liquids tec Heat Capacity In Calorimetry the calorimetry calculator can help you solve complex calorimetry problems. Calorimetry is used to measure. It can analyze the heat exchange between up to 3 objects. when analysing a heat transfer reaction, chemists use the calorimetry equation relating heat released in the reaction to the substance’s mass, change in. The specific heat capacity (c) of a substance is. Heat Capacity In Calorimetry.
From www.showme.com
Calculating Heat Capacity Heat Transfer, Heat Capacity, Specific Heat Heat Capacity In Calorimetry q = mcδt, where q is the symbol for heat transfer (“quantity of heat”), m is the mass of the substance, and δt is the change in temperature. one technique we can use to measure the amount of heat involved in a chemical or physical process is known as calorimetry. the calorimetry calculator can help you solve. Heat Capacity In Calorimetry.
From www.sliderbase.com
Basic Thermochemistry Presentation Chemistry Heat Capacity In Calorimetry the calorimetry calculator can help you solve complex calorimetry problems. q = mcδt, where q is the symbol for heat transfer (“quantity of heat”), m is the mass of the substance, and δt is the change in temperature. It can analyze the heat exchange between up to 3 objects. Calorimetry is used to measure. when analysing a. Heat Capacity In Calorimetry.
From www.expii.com
Heat Capacity of Water — Overview & Importance Expii Heat Capacity In Calorimetry a calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. the calorimetry calculator can help you solve complex calorimetry problems. It can analyze the heat exchange between up to 3 objects. Calorimetry is used to measure. one technique we can use to measure the amount of heat involved. Heat Capacity In Calorimetry.
From www.slideserve.com
PPT Section 7.2—Calorimetry & Heat Capacity PowerPoint Presentation Heat Capacity In Calorimetry the calorimetry calculator can help you solve complex calorimetry problems. one technique we can use to measure the amount of heat involved in a chemical or physical process is known as calorimetry. It can analyze the heat exchange between up to 3 objects. a calorimeter is a device used to measure the amount of heat involved in. Heat Capacity In Calorimetry.
From www.youtube.com
Specific heat capacity YouTube Heat Capacity In Calorimetry q = mcδt, where q is the symbol for heat transfer (“quantity of heat”), m is the mass of the substance, and δt is the change in temperature. The specific heat capacity (c) of a substance is the heating required to raise the temperature of 1 g of a. the heat capacity of the calorimeter or of the. Heat Capacity In Calorimetry.
From www.slideshare.net
Tang 01 heat capacity and calorimetry Heat Capacity In Calorimetry the calorimetry calculator can help you solve complex calorimetry problems. Calorimetry is used to measure. The specific heat capacity (c) of a substance is the heating required to raise the temperature of 1 g of a. q = mcδt, where q is the symbol for heat transfer (“quantity of heat”), m is the mass of the substance, and. Heat Capacity In Calorimetry.
From www.youtube.com
AP Chemistry Thermochemical Equations and Calorimetry YouTube Heat Capacity In Calorimetry a calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. one technique we can use to measure the amount of heat involved in a chemical or physical process is known as calorimetry. the calorimetry calculator can help you solve complex calorimetry problems. Calorimetry is used to measure. It. Heat Capacity In Calorimetry.
From www.slideserve.com
PPT Chapter 8 PowerPoint Presentation, free download ID6933946 Heat Capacity In Calorimetry It can analyze the heat exchange between up to 3 objects. when analysing a heat transfer reaction, chemists use the calorimetry equation relating heat released in the reaction to the substance’s mass, change in. the calorimetry calculator can help you solve complex calorimetry problems. the heat capacity of the calorimeter or of the reaction mixture may be. Heat Capacity In Calorimetry.
From 2012books.lardbucket.org
Calorimetry Heat Capacity In Calorimetry when analysing a heat transfer reaction, chemists use the calorimetry equation relating heat released in the reaction to the substance’s mass, change in. Calorimetry is used to measure. q = mcδt, where q is the symbol for heat transfer (“quantity of heat”), m is the mass of the substance, and δt is the change in temperature. It can. Heat Capacity In Calorimetry.
From dxowceosf.blob.core.windows.net
Calorimetry All Formulas at Spencer McSwain blog Heat Capacity In Calorimetry the heat capacity of the calorimeter or of the reaction mixture may be used to calculate the amount of heat released or absorbed. a calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. Calorimetry is used to measure. The specific heat capacity (c) of a substance is the heating. Heat Capacity In Calorimetry.
From www.slideshare.net
Tang 01 heat capacity and calorimetry Heat Capacity In Calorimetry The specific heat capacity (c) of a substance is the heating required to raise the temperature of 1 g of a. q = mcδt, where q is the symbol for heat transfer (“quantity of heat”), m is the mass of the substance, and δt is the change in temperature. a calorimeter is a device used to measure the. Heat Capacity In Calorimetry.
From chem.libretexts.org
5.3 Calorimetry Chemistry LibreTexts Heat Capacity In Calorimetry the heat capacity of the calorimeter or of the reaction mixture may be used to calculate the amount of heat released or absorbed. the calorimetry calculator can help you solve complex calorimetry problems. It can analyze the heat exchange between up to 3 objects. The specific heat capacity (c) of a substance is the heating required to raise. Heat Capacity In Calorimetry.
From www.chegg.com
Solved CALORIMETRY HEAT CAPACITY OF A CALORIMETER Heat Capacity In Calorimetry the heat capacity of the calorimeter or of the reaction mixture may be used to calculate the amount of heat released or absorbed. The specific heat capacity (c) of a substance is the heating required to raise the temperature of 1 g of a. Calorimetry is used to measure. one technique we can use to measure the amount. Heat Capacity In Calorimetry.
From www.slideshare.net
Tang 01 heat capacity and calorimetry Heat Capacity In Calorimetry the heat capacity of the calorimeter or of the reaction mixture may be used to calculate the amount of heat released or absorbed. Calorimetry is used to measure. It can analyze the heat exchange between up to 3 objects. the calorimetry calculator can help you solve complex calorimetry problems. when analysing a heat transfer reaction, chemists use. Heat Capacity In Calorimetry.
From slideplayer.com
Specific Heat Capacity & Calorimetry ppt download Heat Capacity In Calorimetry when analysing a heat transfer reaction, chemists use the calorimetry equation relating heat released in the reaction to the substance’s mass, change in. the calorimetry calculator can help you solve complex calorimetry problems. Calorimetry is used to measure. The specific heat capacity (c) of a substance is the heating required to raise the temperature of 1 g of. Heat Capacity In Calorimetry.
From ecampusontario.pressbooks.pub
5.2 Calorimetry Chemistry Heat Capacity In Calorimetry Calorimetry is used to measure. the calorimetry calculator can help you solve complex calorimetry problems. a calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. the heat capacity of the calorimeter or of the reaction mixture may be used to calculate the amount of heat released or absorbed.. Heat Capacity In Calorimetry.
From www.slideserve.com
PPT Calorimetry PowerPoint Presentation ID6655927 Heat Capacity In Calorimetry the heat capacity of the calorimeter or of the reaction mixture may be used to calculate the amount of heat released or absorbed. The specific heat capacity (c) of a substance is the heating required to raise the temperature of 1 g of a. when analysing a heat transfer reaction, chemists use the calorimetry equation relating heat released. Heat Capacity In Calorimetry.
From www.scribd.com
Calorimetry Lab Heat Capacity Heat Heat Capacity In Calorimetry Calorimetry is used to measure. one technique we can use to measure the amount of heat involved in a chemical or physical process is known as calorimetry. q = mcδt, where q is the symbol for heat transfer (“quantity of heat”), m is the mass of the substance, and δt is the change in temperature. when analysing. Heat Capacity In Calorimetry.
From edurev.in
Calorimetry and Specific Heat Capacity Class 11 Notes EduRev Heat Capacity In Calorimetry one technique we can use to measure the amount of heat involved in a chemical or physical process is known as calorimetry. The specific heat capacity (c) of a substance is the heating required to raise the temperature of 1 g of a. Calorimetry is used to measure. q = mcδt, where q is the symbol for heat. Heat Capacity In Calorimetry.
From thechemistrynotes.com
Calorimetry Definition, Principle, Types, Application, and Limitations Heat Capacity In Calorimetry the heat capacity of the calorimeter or of the reaction mixture may be used to calculate the amount of heat released or absorbed. the calorimetry calculator can help you solve complex calorimetry problems. Calorimetry is used to measure. one technique we can use to measure the amount of heat involved in a chemical or physical process is. Heat Capacity In Calorimetry.
From www.slideshare.net
Tang 01 heat capacity and calorimetry Heat Capacity In Calorimetry one technique we can use to measure the amount of heat involved in a chemical or physical process is known as calorimetry. when analysing a heat transfer reaction, chemists use the calorimetry equation relating heat released in the reaction to the substance’s mass, change in. the calorimetry calculator can help you solve complex calorimetry problems. q. Heat Capacity In Calorimetry.
From courses.lumenlearning.com
Calorimetry Chemistry for Majors Heat Capacity In Calorimetry the calorimetry calculator can help you solve complex calorimetry problems. q = mcδt, where q is the symbol for heat transfer (“quantity of heat”), m is the mass of the substance, and δt is the change in temperature. a calorimeter is a device used to measure the amount of heat involved in a chemical or physical process.. Heat Capacity In Calorimetry.
From www.youtube.com
CHEMISTRY 101 Calculating Heat Capacity of a Bomb Calorimeter YouTube Heat Capacity In Calorimetry when analysing a heat transfer reaction, chemists use the calorimetry equation relating heat released in the reaction to the substance’s mass, change in. The specific heat capacity (c) of a substance is the heating required to raise the temperature of 1 g of a. the heat capacity of the calorimeter or of the reaction mixture may be used. Heat Capacity In Calorimetry.
From www.youtube.com
050 Calorimetry YouTube Heat Capacity In Calorimetry Calorimetry is used to measure. a calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. when analysing a heat transfer reaction, chemists use the calorimetry equation relating heat released in the reaction to the substance’s mass, change in. the heat capacity of the calorimeter or of the reaction. Heat Capacity In Calorimetry.
From www.slideshare.net
Tang 01 heat capacity and calorimetry Heat Capacity In Calorimetry the heat capacity of the calorimeter or of the reaction mixture may be used to calculate the amount of heat released or absorbed. It can analyze the heat exchange between up to 3 objects. Calorimetry is used to measure. the calorimetry calculator can help you solve complex calorimetry problems. The specific heat capacity (c) of a substance is. Heat Capacity In Calorimetry.
From www.slideserve.com
PPT Section 7.2—Calorimetry & Heat Capacity PowerPoint Presentation Heat Capacity In Calorimetry q = mcδt, where q is the symbol for heat transfer (“quantity of heat”), m is the mass of the substance, and δt is the change in temperature. one technique we can use to measure the amount of heat involved in a chemical or physical process is known as calorimetry. a calorimeter is a device used to. Heat Capacity In Calorimetry.
From www.youtube.com
Energy 5 Calorimetry/Specific Heat Lab YouTube Heat Capacity In Calorimetry Calorimetry is used to measure. a calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. one technique we can use to measure the amount of heat involved in a chemical or physical process is known as calorimetry. the calorimetry calculator can help you solve complex calorimetry problems. . Heat Capacity In Calorimetry.