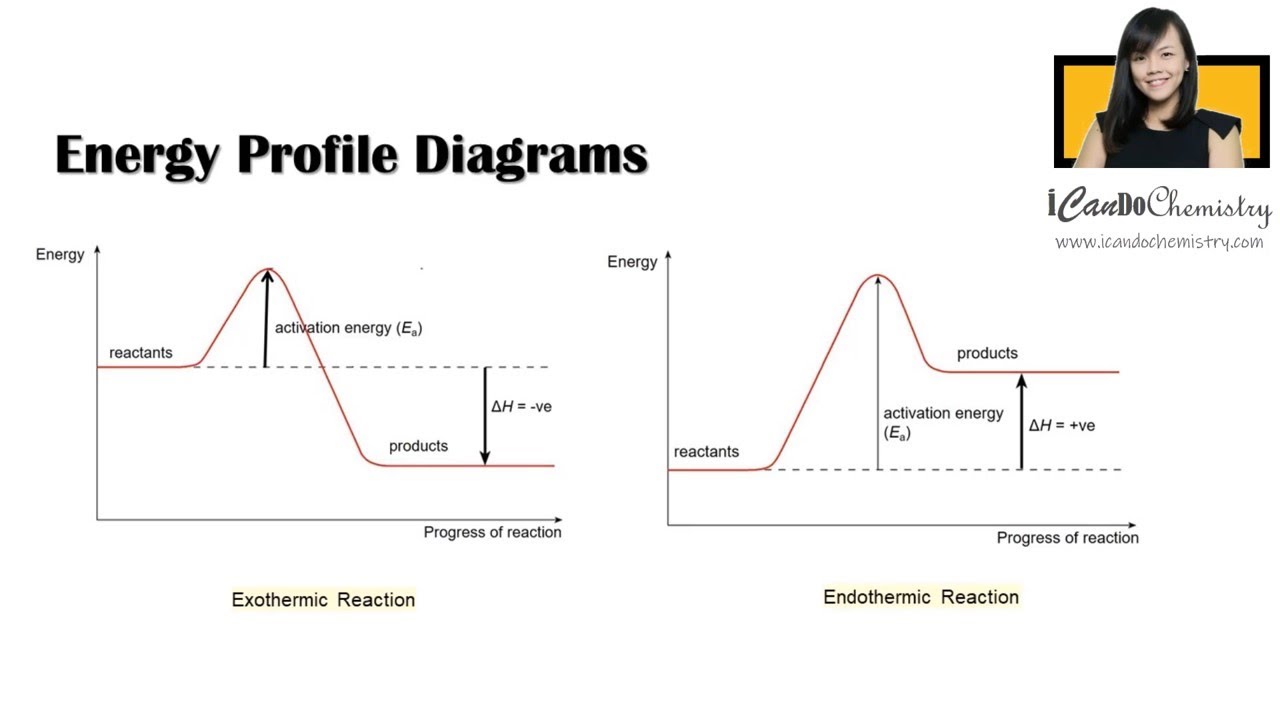
Endothermic Reaction Graph Activation Energy . Learn the energy diagram and check out a few examples. This is asking you to draw a potential energy diagram for an endothermic reaction. Some chemical reactions need a constant input of energy to take place. The activation energy (ea), labeled δg ‡ in figure 2, is the energy difference between the reactants and the activated complex, also known as. The energy level increases in an endothermic reaction. Reactions which have higher activation energies require more energy to start than those with lower activation energies. Compare and contrast endothermic and exothermic reactions. This is because energy is taken in from the surroundings. We can graph the energy of a reaction by plotting the potential energy of the system as the reaction progresses. Find out about the endothermic reaction. They are called endothermic reactions. Recall that deltah_rxn, the enthalpy of reaction, is positive for endothermic reactions, i.e. Activating endothermic and exothermic reactions.
from www.youtube.com
Some chemical reactions need a constant input of energy to take place. Find out about the endothermic reaction. This is because energy is taken in from the surroundings. This is asking you to draw a potential energy diagram for an endothermic reaction. Recall that deltah_rxn, the enthalpy of reaction, is positive for endothermic reactions, i.e. Reactions which have higher activation energies require more energy to start than those with lower activation energies. Compare and contrast endothermic and exothermic reactions. They are called endothermic reactions. The activation energy (ea), labeled δg ‡ in figure 2, is the energy difference between the reactants and the activated complex, also known as. Activating endothermic and exothermic reactions.
How to draw Energy Profile Diagram and Energy Level Diagram of
Endothermic Reaction Graph Activation Energy We can graph the energy of a reaction by plotting the potential energy of the system as the reaction progresses. This is because energy is taken in from the surroundings. Activating endothermic and exothermic reactions. Compare and contrast endothermic and exothermic reactions. Learn the energy diagram and check out a few examples. This is asking you to draw a potential energy diagram for an endothermic reaction. The activation energy (ea), labeled δg ‡ in figure 2, is the energy difference between the reactants and the activated complex, also known as. We can graph the energy of a reaction by plotting the potential energy of the system as the reaction progresses. Some chemical reactions need a constant input of energy to take place. Find out about the endothermic reaction. The energy level increases in an endothermic reaction. They are called endothermic reactions. Reactions which have higher activation energies require more energy to start than those with lower activation energies. Recall that deltah_rxn, the enthalpy of reaction, is positive for endothermic reactions, i.e.
From www.coursehero.com
[Solved] /. Is this an endothermic or exothermic graph? Energy Diagram Endothermic Reaction Graph Activation Energy We can graph the energy of a reaction by plotting the potential energy of the system as the reaction progresses. Recall that deltah_rxn, the enthalpy of reaction, is positive for endothermic reactions, i.e. Activating endothermic and exothermic reactions. This is asking you to draw a potential energy diagram for an endothermic reaction. They are called endothermic reactions. The energy level. Endothermic Reaction Graph Activation Energy.
From stock.adobe.com
Activation energy in endothermic and exothermic reactions. Stock Endothermic Reaction Graph Activation Energy Reactions which have higher activation energies require more energy to start than those with lower activation energies. We can graph the energy of a reaction by plotting the potential energy of the system as the reaction progresses. Find out about the endothermic reaction. Compare and contrast endothermic and exothermic reactions. Learn the energy diagram and check out a few examples.. Endothermic Reaction Graph Activation Energy.
From schematicmandorlas.z14.web.core.windows.net
Energy Diagram For An Endothermic Reaction Endothermic Reaction Graph Activation Energy They are called endothermic reactions. The activation energy (ea), labeled δg ‡ in figure 2, is the energy difference between the reactants and the activated complex, also known as. We can graph the energy of a reaction by plotting the potential energy of the system as the reaction progresses. Activating endothermic and exothermic reactions. This is because energy is taken. Endothermic Reaction Graph Activation Energy.
From professional.patrickneyman.com
Activation Energy Equation Endothermic Reaction Graph Activation Energy Compare and contrast endothermic and exothermic reactions. Recall that deltah_rxn, the enthalpy of reaction, is positive for endothermic reactions, i.e. Learn the energy diagram and check out a few examples. Activating endothermic and exothermic reactions. They are called endothermic reactions. Some chemical reactions need a constant input of energy to take place. Find out about the endothermic reaction. The activation. Endothermic Reaction Graph Activation Energy.
From www.youtube.com
How to draw Energy Profile Diagram and Energy Level Diagram of Endothermic Reaction Graph Activation Energy Recall that deltah_rxn, the enthalpy of reaction, is positive for endothermic reactions, i.e. Compare and contrast endothermic and exothermic reactions. This is because energy is taken in from the surroundings. Some chemical reactions need a constant input of energy to take place. Learn the energy diagram and check out a few examples. We can graph the energy of a reaction. Endothermic Reaction Graph Activation Energy.
From stock.adobe.com
endothermic reaction graph in chemistry Stock Vector Adobe Stock Endothermic Reaction Graph Activation Energy Reactions which have higher activation energies require more energy to start than those with lower activation energies. The activation energy (ea), labeled δg ‡ in figure 2, is the energy difference between the reactants and the activated complex, also known as. Learn the energy diagram and check out a few examples. Find out about the endothermic reaction. Some chemical reactions. Endothermic Reaction Graph Activation Energy.
From www.istockphoto.com
Vector Graphs Of Endothermic And Exothermic Reactions Activation Energy Endothermic Reaction Graph Activation Energy The activation energy (ea), labeled δg ‡ in figure 2, is the energy difference between the reactants and the activated complex, also known as. Activating endothermic and exothermic reactions. Recall that deltah_rxn, the enthalpy of reaction, is positive for endothermic reactions, i.e. Compare and contrast endothermic and exothermic reactions. The energy level increases in an endothermic reaction. They are called. Endothermic Reaction Graph Activation Energy.
From diagramlibwegwchjx5.z13.web.core.windows.net
Enthalpy Diagram For Endothermic Reaction Endothermic Reaction Graph Activation Energy Learn the energy diagram and check out a few examples. Find out about the endothermic reaction. The activation energy (ea), labeled δg ‡ in figure 2, is the energy difference between the reactants and the activated complex, also known as. This is because energy is taken in from the surroundings. Activating endothermic and exothermic reactions. They are called endothermic reactions.. Endothermic Reaction Graph Activation Energy.
From www.studyorgo.com
How to Interpret Thermodynamics of Reactions Endothermic Reaction Graph Activation Energy This is because energy is taken in from the surroundings. The activation energy (ea), labeled δg ‡ in figure 2, is the energy difference between the reactants and the activated complex, also known as. We can graph the energy of a reaction by plotting the potential energy of the system as the reaction progresses. This is asking you to draw. Endothermic Reaction Graph Activation Energy.
From www.dreamstime.com
Activation Energy Endothermic Reaction 2d Illustration Stock Endothermic Reaction Graph Activation Energy Activating endothermic and exothermic reactions. We can graph the energy of a reaction by plotting the potential energy of the system as the reaction progresses. Some chemical reactions need a constant input of energy to take place. Compare and contrast endothermic and exothermic reactions. Recall that deltah_rxn, the enthalpy of reaction, is positive for endothermic reactions, i.e. They are called. Endothermic Reaction Graph Activation Energy.
From byjus.com
Activation Energy Definition, Formula, SI Units, Examples, Calculation Endothermic Reaction Graph Activation Energy Activating endothermic and exothermic reactions. Some chemical reactions need a constant input of energy to take place. Learn the energy diagram and check out a few examples. Recall that deltah_rxn, the enthalpy of reaction, is positive for endothermic reactions, i.e. The activation energy (ea), labeled δg ‡ in figure 2, is the energy difference between the reactants and the activated. Endothermic Reaction Graph Activation Energy.
From telegra.ph
Endothermic energy profile diagram activation Telegraph Endothermic Reaction Graph Activation Energy Activating endothermic and exothermic reactions. Find out about the endothermic reaction. Some chemical reactions need a constant input of energy to take place. Reactions which have higher activation energies require more energy to start than those with lower activation energies. They are called endothermic reactions. Recall that deltah_rxn, the enthalpy of reaction, is positive for endothermic reactions, i.e. The activation. Endothermic Reaction Graph Activation Energy.
From schematicdbelfriede.z13.web.core.windows.net
Energy Diagram For Endothermic Reaction Endothermic Reaction Graph Activation Energy Compare and contrast endothermic and exothermic reactions. Find out about the endothermic reaction. This is asking you to draw a potential energy diagram for an endothermic reaction. They are called endothermic reactions. Recall that deltah_rxn, the enthalpy of reaction, is positive for endothermic reactions, i.e. The activation energy (ea), labeled δg ‡ in figure 2, is the energy difference between. Endothermic Reaction Graph Activation Energy.
From www.varsitytutors.com
Reaction Coordinate Diagrams College Chemistry Endothermic Reaction Graph Activation Energy Find out about the endothermic reaction. Activating endothermic and exothermic reactions. This is because energy is taken in from the surroundings. The energy level increases in an endothermic reaction. Reactions which have higher activation energies require more energy to start than those with lower activation energies. We can graph the energy of a reaction by plotting the potential energy of. Endothermic Reaction Graph Activation Energy.
From www.youtube.com
Endothermic and Exothermic Reactions With Potential Energy Diagrams Endothermic Reaction Graph Activation Energy Activating endothermic and exothermic reactions. The energy level increases in an endothermic reaction. Learn the energy diagram and check out a few examples. They are called endothermic reactions. Some chemical reactions need a constant input of energy to take place. Recall that deltah_rxn, the enthalpy of reaction, is positive for endothermic reactions, i.e. This is asking you to draw a. Endothermic Reaction Graph Activation Energy.
From stock.adobe.com
activation energy endothermic reaction diagram vector de Stock Adobe Endothermic Reaction Graph Activation Energy Learn the energy diagram and check out a few examples. Reactions which have higher activation energies require more energy to start than those with lower activation energies. We can graph the energy of a reaction by plotting the potential energy of the system as the reaction progresses. Some chemical reactions need a constant input of energy to take place. Activating. Endothermic Reaction Graph Activation Energy.
From www.doubtnut.com
For an endothermic reaction energy of activation is E(a) and enthlpy o Endothermic Reaction Graph Activation Energy This is asking you to draw a potential energy diagram for an endothermic reaction. The activation energy (ea), labeled δg ‡ in figure 2, is the energy difference between the reactants and the activated complex, also known as. Compare and contrast endothermic and exothermic reactions. We can graph the energy of a reaction by plotting the potential energy of the. Endothermic Reaction Graph Activation Energy.
From printablebrebalgek89.z4.web.core.windows.net
Endothermic And Exothermic Explained Endothermic Reaction Graph Activation Energy Activating endothermic and exothermic reactions. Recall that deltah_rxn, the enthalpy of reaction, is positive for endothermic reactions, i.e. This is because energy is taken in from the surroundings. Some chemical reactions need a constant input of energy to take place. Reactions which have higher activation energies require more energy to start than those with lower activation energies. The activation energy. Endothermic Reaction Graph Activation Energy.
From www.slideserve.com
PPT Endothermic Vs. Exothermic Reaction Graphs PowerPoint Endothermic Reaction Graph Activation Energy Recall that deltah_rxn, the enthalpy of reaction, is positive for endothermic reactions, i.e. The activation energy (ea), labeled δg ‡ in figure 2, is the energy difference between the reactants and the activated complex, also known as. Reactions which have higher activation energies require more energy to start than those with lower activation energies. The energy level increases in an. Endothermic Reaction Graph Activation Energy.
From byjus.com
24. What is the Minimum activation energy required for endothermic and Endothermic Reaction Graph Activation Energy They are called endothermic reactions. Activating endothermic and exothermic reactions. Reactions which have higher activation energies require more energy to start than those with lower activation energies. Some chemical reactions need a constant input of energy to take place. We can graph the energy of a reaction by plotting the potential energy of the system as the reaction progresses. Compare. Endothermic Reaction Graph Activation Energy.
From www.kosmotime.com
Activation Energy The Secret to Getting Started and Getting Finished Endothermic Reaction Graph Activation Energy Recall that deltah_rxn, the enthalpy of reaction, is positive for endothermic reactions, i.e. They are called endothermic reactions. Reactions which have higher activation energies require more energy to start than those with lower activation energies. We can graph the energy of a reaction by plotting the potential energy of the system as the reaction progresses. Learn the energy diagram and. Endothermic Reaction Graph Activation Energy.
From revisechemistry.uk
Exothermic and Endothermic Reactions AQA C5 revisechemistry.uk Endothermic Reaction Graph Activation Energy The energy level increases in an endothermic reaction. Some chemical reactions need a constant input of energy to take place. This is because energy is taken in from the surroundings. Compare and contrast endothermic and exothermic reactions. Learn the energy diagram and check out a few examples. Recall that deltah_rxn, the enthalpy of reaction, is positive for endothermic reactions, i.e.. Endothermic Reaction Graph Activation Energy.
From www.doubtnut.com
An endothermic reaction with high activation energy for the forward re Endothermic Reaction Graph Activation Energy Recall that deltah_rxn, the enthalpy of reaction, is positive for endothermic reactions, i.e. The energy level increases in an endothermic reaction. Find out about the endothermic reaction. Compare and contrast endothermic and exothermic reactions. This is asking you to draw a potential energy diagram for an endothermic reaction. The activation energy (ea), labeled δg ‡ in figure 2, is the. Endothermic Reaction Graph Activation Energy.
From manualpartsynaxis123.z13.web.core.windows.net
Exothermic And Endothermic Energy Diagrams Endothermic Reaction Graph Activation Energy Some chemical reactions need a constant input of energy to take place. We can graph the energy of a reaction by plotting the potential energy of the system as the reaction progresses. Recall that deltah_rxn, the enthalpy of reaction, is positive for endothermic reactions, i.e. The activation energy (ea), labeled δg ‡ in figure 2, is the energy difference between. Endothermic Reaction Graph Activation Energy.
From www.slideserve.com
PPT Endothermic Vs. Exothermic Reaction Graphs PowerPoint Endothermic Reaction Graph Activation Energy Activating endothermic and exothermic reactions. Reactions which have higher activation energies require more energy to start than those with lower activation energies. This is asking you to draw a potential energy diagram for an endothermic reaction. We can graph the energy of a reaction by plotting the potential energy of the system as the reaction progresses. Recall that deltah_rxn, the. Endothermic Reaction Graph Activation Energy.
From online-learning-college.com
Energy level diagrams Endothermic & Exothermic reactions Endothermic Reaction Graph Activation Energy Compare and contrast endothermic and exothermic reactions. This is because energy is taken in from the surroundings. Some chemical reactions need a constant input of energy to take place. Reactions which have higher activation energies require more energy to start than those with lower activation energies. The activation energy (ea), labeled δg ‡ in figure 2, is the energy difference. Endothermic Reaction Graph Activation Energy.
From www.slideserve.com
PPT Chapter 7 PowerPoint Presentation ID239015 Endothermic Reaction Graph Activation Energy Find out about the endothermic reaction. They are called endothermic reactions. Learn the energy diagram and check out a few examples. Reactions which have higher activation energies require more energy to start than those with lower activation energies. Recall that deltah_rxn, the enthalpy of reaction, is positive for endothermic reactions, i.e. Compare and contrast endothermic and exothermic reactions. This is. Endothermic Reaction Graph Activation Energy.
From socratic.org
What are activation energies? Socratic Endothermic Reaction Graph Activation Energy We can graph the energy of a reaction by plotting the potential energy of the system as the reaction progresses. Reactions which have higher activation energies require more energy to start than those with lower activation energies. The activation energy (ea), labeled δg ‡ in figure 2, is the energy difference between the reactants and the activated complex, also known. Endothermic Reaction Graph Activation Energy.
From www.youtube.com
ACTIVATION ENERGY WITH GRAPH FOR EXOTHERMIC AND ENDOTHERMIC REACTION L1 Endothermic Reaction Graph Activation Energy Some chemical reactions need a constant input of energy to take place. This is because energy is taken in from the surroundings. Recall that deltah_rxn, the enthalpy of reaction, is positive for endothermic reactions, i.e. The activation energy (ea), labeled δg ‡ in figure 2, is the energy difference between the reactants and the activated complex, also known as. They. Endothermic Reaction Graph Activation Energy.
From www.expii.com
Energy Diagram — Overview & Parts Expii Endothermic Reaction Graph Activation Energy We can graph the energy of a reaction by plotting the potential energy of the system as the reaction progresses. Compare and contrast endothermic and exothermic reactions. The activation energy (ea), labeled δg ‡ in figure 2, is the energy difference between the reactants and the activated complex, also known as. This is because energy is taken in from the. Endothermic Reaction Graph Activation Energy.
From spmchemistry.blog.onlinetuition.com.my
Collision Theory SPM Chemistry Endothermic Reaction Graph Activation Energy They are called endothermic reactions. This is because energy is taken in from the surroundings. Find out about the endothermic reaction. The energy level increases in an endothermic reaction. Learn the energy diagram and check out a few examples. The activation energy (ea), labeled δg ‡ in figure 2, is the energy difference between the reactants and the activated complex,. Endothermic Reaction Graph Activation Energy.
From circuitdiagramtween.z22.web.core.windows.net
Energy Diagram Exothermic Reaction Endothermic Reaction Graph Activation Energy Compare and contrast endothermic and exothermic reactions. This is asking you to draw a potential energy diagram for an endothermic reaction. Find out about the endothermic reaction. Some chemical reactions need a constant input of energy to take place. The energy level increases in an endothermic reaction. This is because energy is taken in from the surroundings. We can graph. Endothermic Reaction Graph Activation Energy.
From robhosking.com
10+ Endothermic Energy Diagram Robhosking Diagram Endothermic Reaction Graph Activation Energy The activation energy (ea), labeled δg ‡ in figure 2, is the energy difference between the reactants and the activated complex, also known as. Compare and contrast endothermic and exothermic reactions. They are called endothermic reactions. Find out about the endothermic reaction. We can graph the energy of a reaction by plotting the potential energy of the system as the. Endothermic Reaction Graph Activation Energy.
From byjus.com
Activation Energy Definition, Formula, SI Units, Examples, Calculation Endothermic Reaction Graph Activation Energy We can graph the energy of a reaction by plotting the potential energy of the system as the reaction progresses. Recall that deltah_rxn, the enthalpy of reaction, is positive for endothermic reactions, i.e. Activating endothermic and exothermic reactions. The energy level increases in an endothermic reaction. Compare and contrast endothermic and exothermic reactions. Reactions which have higher activation energies require. Endothermic Reaction Graph Activation Energy.
From www.tutormyself.com
314 (Triple only) draw and explain reaction profile diagrams showing Endothermic Reaction Graph Activation Energy This is asking you to draw a potential energy diagram for an endothermic reaction. Find out about the endothermic reaction. This is because energy is taken in from the surroundings. We can graph the energy of a reaction by plotting the potential energy of the system as the reaction progresses. The activation energy (ea), labeled δg ‡ in figure 2,. Endothermic Reaction Graph Activation Energy.