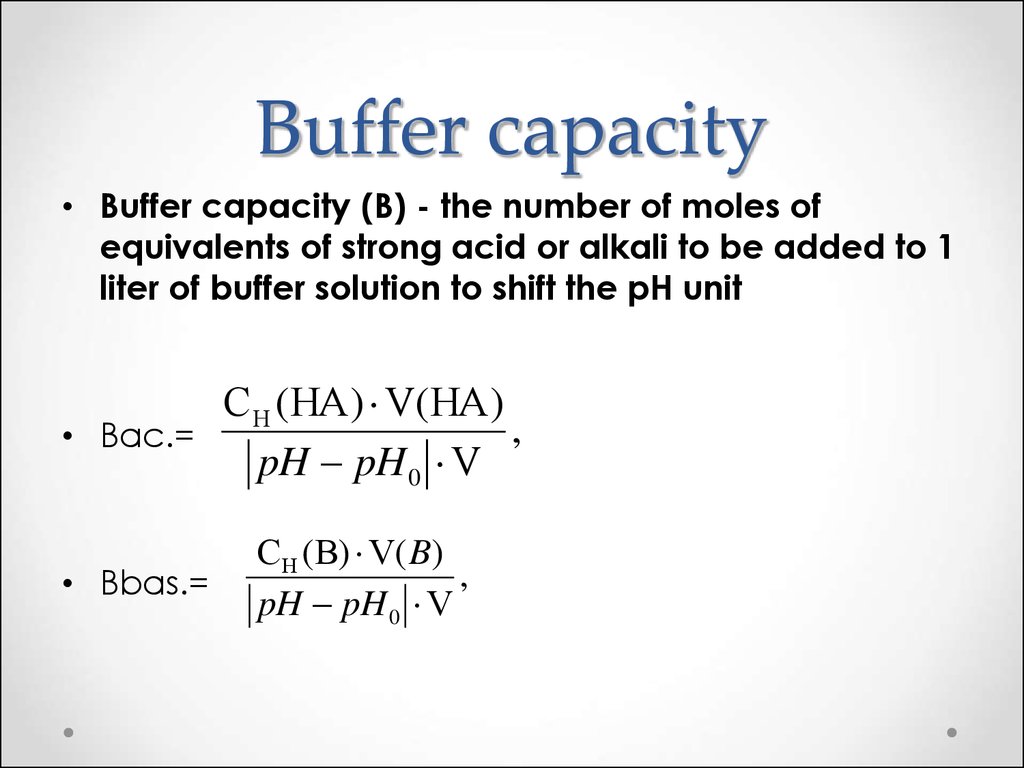
What Is Higher Buffer Capacity Mean . A buffer has its highest capacity when the ph is equal to the pka of its acidic component. The capacity of a buffer and its initial ph impact the ph change after the addition of an. The buffer capacity is a measure of resistant a particular solution is resistant to change in ph when an acid or a base is added to it. On the other hand, buffer. Buffer solutions have a working ph range and capacity which dictate how much acid/base can be neutralized before ph changes, and the amount by. Buffer capacity refers to the ability of a buffer solution to resist changes in its ph when an acid or base is added. If you remember high school chemistry or took a college course like chemistry 101, you will have conducted a titration test. Buffers are characterized by the ph range over which they can maintain a more or less constant ph and by their buffer capacity, the amount of strong acid or base that can be absorbed before the ph changes significantly. Buffer capacity decreases significantly when the ph is.
from en.ppt-online.org
The buffer capacity is a measure of resistant a particular solution is resistant to change in ph when an acid or a base is added to it. Buffer solutions have a working ph range and capacity which dictate how much acid/base can be neutralized before ph changes, and the amount by. Buffers are characterized by the ph range over which they can maintain a more or less constant ph and by their buffer capacity, the amount of strong acid or base that can be absorbed before the ph changes significantly. On the other hand, buffer. Buffer capacity refers to the ability of a buffer solution to resist changes in its ph when an acid or base is added. If you remember high school chemistry or took a college course like chemistry 101, you will have conducted a titration test. The capacity of a buffer and its initial ph impact the ph change after the addition of an. Buffer capacity decreases significantly when the ph is. A buffer has its highest capacity when the ph is equal to the pka of its acidic component.
Solutions. Acidbase equilibrium in biological systems online
What Is Higher Buffer Capacity Mean A buffer has its highest capacity when the ph is equal to the pka of its acidic component. On the other hand, buffer. Buffer capacity decreases significantly when the ph is. Buffer capacity refers to the ability of a buffer solution to resist changes in its ph when an acid or base is added. Buffer solutions have a working ph range and capacity which dictate how much acid/base can be neutralized before ph changes, and the amount by. The capacity of a buffer and its initial ph impact the ph change after the addition of an. Buffers are characterized by the ph range over which they can maintain a more or less constant ph and by their buffer capacity, the amount of strong acid or base that can be absorbed before the ph changes significantly. If you remember high school chemistry or took a college course like chemistry 101, you will have conducted a titration test. The buffer capacity is a measure of resistant a particular solution is resistant to change in ph when an acid or a base is added to it. A buffer has its highest capacity when the ph is equal to the pka of its acidic component.
From www.slideserve.com
PPT Unit 6 Chpt 15 Acid/Base Equilibria PowerPoint Presentation What Is Higher Buffer Capacity Mean The capacity of a buffer and its initial ph impact the ph change after the addition of an. The buffer capacity is a measure of resistant a particular solution is resistant to change in ph when an acid or a base is added to it. Buffers are characterized by the ph range over which they can maintain a more or. What Is Higher Buffer Capacity Mean.
From www.slideserve.com
PPT Chapter 16 Aqueous Ionic Equilibrium PowerPoint Presentation What Is Higher Buffer Capacity Mean The capacity of a buffer and its initial ph impact the ph change after the addition of an. Buffer capacity refers to the ability of a buffer solution to resist changes in its ph when an acid or base is added. Buffer solutions have a working ph range and capacity which dictate how much acid/base can be neutralized before ph. What Is Higher Buffer Capacity Mean.
From chemexpert.net
Mastering Buffer Capacity Your Guide To Maintaining Chemical Harmony What Is Higher Buffer Capacity Mean Buffer solutions have a working ph range and capacity which dictate how much acid/base can be neutralized before ph changes, and the amount by. If you remember high school chemistry or took a college course like chemistry 101, you will have conducted a titration test. Buffer capacity refers to the ability of a buffer solution to resist changes in its. What Is Higher Buffer Capacity Mean.
From www.researchgate.net
6. Schematic diagram of the buffer capacity of an aqueous system What Is Higher Buffer Capacity Mean On the other hand, buffer. Buffer solutions have a working ph range and capacity which dictate how much acid/base can be neutralized before ph changes, and the amount by. The capacity of a buffer and its initial ph impact the ph change after the addition of an. The buffer capacity is a measure of resistant a particular solution is resistant. What Is Higher Buffer Capacity Mean.
From www.slideserve.com
PPT Buffer Capacity Complexation PowerPoint Presentation, free What Is Higher Buffer Capacity Mean If you remember high school chemistry or took a college course like chemistry 101, you will have conducted a titration test. Buffer solutions have a working ph range and capacity which dictate how much acid/base can be neutralized before ph changes, and the amount by. Buffer capacity decreases significantly when the ph is. Buffer capacity refers to the ability of. What Is Higher Buffer Capacity Mean.
From www.slideserve.com
PPT Buffers & Buffer Capacity PowerPoint Presentation ID5949383 What Is Higher Buffer Capacity Mean Buffer solutions have a working ph range and capacity which dictate how much acid/base can be neutralized before ph changes, and the amount by. If you remember high school chemistry or took a college course like chemistry 101, you will have conducted a titration test. A buffer has its highest capacity when the ph is equal to the pka of. What Is Higher Buffer Capacity Mean.
From www.slideserve.com
PPT Buffering Capacity Addition of STRONG Acids or Bases PowerPoint What Is Higher Buffer Capacity Mean Buffer capacity refers to the ability of a buffer solution to resist changes in its ph when an acid or base is added. Buffer solutions have a working ph range and capacity which dictate how much acid/base can be neutralized before ph changes, and the amount by. The capacity of a buffer and its initial ph impact the ph change. What Is Higher Buffer Capacity Mean.
From www.slideserve.com
PPT Chapter 15 PowerPoint Presentation, free download ID4347030 What Is Higher Buffer Capacity Mean The capacity of a buffer and its initial ph impact the ph change after the addition of an. Buffer capacity decreases significantly when the ph is. Buffer solutions have a working ph range and capacity which dictate how much acid/base can be neutralized before ph changes, and the amount by. If you remember high school chemistry or took a college. What Is Higher Buffer Capacity Mean.
From www.engineeringtoolbox.com
Buffer solutions What Is Higher Buffer Capacity Mean The buffer capacity is a measure of resistant a particular solution is resistant to change in ph when an acid or a base is added to it. The capacity of a buffer and its initial ph impact the ph change after the addition of an. On the other hand, buffer. Buffer capacity refers to the ability of a buffer solution. What Is Higher Buffer Capacity Mean.
From www.slideserve.com
PPT Chapter 8 & 9 PowerPoint Presentation, free download ID991133 What Is Higher Buffer Capacity Mean Buffer solutions have a working ph range and capacity which dictate how much acid/base can be neutralized before ph changes, and the amount by. Buffers are characterized by the ph range over which they can maintain a more or less constant ph and by their buffer capacity, the amount of strong acid or base that can be absorbed before the. What Is Higher Buffer Capacity Mean.
From en.ppt-online.org
Solutions. Acidbase equilibrium in biological systems online What Is Higher Buffer Capacity Mean On the other hand, buffer. If you remember high school chemistry or took a college course like chemistry 101, you will have conducted a titration test. A buffer has its highest capacity when the ph is equal to the pka of its acidic component. The capacity of a buffer and its initial ph impact the ph change after the addition. What Is Higher Buffer Capacity Mean.
From www.pinterest.com
Buffer Capacity definition The amount of acid or a base a buffer What Is Higher Buffer Capacity Mean On the other hand, buffer. Buffer capacity decreases significantly when the ph is. Buffers are characterized by the ph range over which they can maintain a more or less constant ph and by their buffer capacity, the amount of strong acid or base that can be absorbed before the ph changes significantly. The buffer capacity is a measure of resistant. What Is Higher Buffer Capacity Mean.
From www.slideserve.com
PPT Chemistry 102(01) Spring 2008 PowerPoint Presentation, free What Is Higher Buffer Capacity Mean On the other hand, buffer. The capacity of a buffer and its initial ph impact the ph change after the addition of an. A buffer has its highest capacity when the ph is equal to the pka of its acidic component. Buffer solutions have a working ph range and capacity which dictate how much acid/base can be neutralized before ph. What Is Higher Buffer Capacity Mean.
From facts.net
13 Enigmatic Facts About Buffer Capacity What Is Higher Buffer Capacity Mean The buffer capacity is a measure of resistant a particular solution is resistant to change in ph when an acid or a base is added to it. Buffers are characterized by the ph range over which they can maintain a more or less constant ph and by their buffer capacity, the amount of strong acid or base that can be. What Is Higher Buffer Capacity Mean.
From www.slideserve.com
PPT Buffering Capacity Addition of STRONG Acids or Bases PowerPoint What Is Higher Buffer Capacity Mean On the other hand, buffer. Buffers are characterized by the ph range over which they can maintain a more or less constant ph and by their buffer capacity, the amount of strong acid or base that can be absorbed before the ph changes significantly. Buffer capacity decreases significantly when the ph is. If you remember high school chemistry or took. What Is Higher Buffer Capacity Mean.
From www.slideserve.com
PPT Chemistry 100 Chapter 17 PowerPoint Presentation, free download What Is Higher Buffer Capacity Mean Buffers are characterized by the ph range over which they can maintain a more or less constant ph and by their buffer capacity, the amount of strong acid or base that can be absorbed before the ph changes significantly. Buffer capacity decreases significantly when the ph is. On the other hand, buffer. Buffer capacity refers to the ability of a. What Is Higher Buffer Capacity Mean.
From thenoveldifference.com
Buffer Action and Buffer Capacity The best 4 difference What Is Higher Buffer Capacity Mean Buffers are characterized by the ph range over which they can maintain a more or less constant ph and by their buffer capacity, the amount of strong acid or base that can be absorbed before the ph changes significantly. The buffer capacity is a measure of resistant a particular solution is resistant to change in ph when an acid or. What Is Higher Buffer Capacity Mean.
From www.youtube.com
Buffer capacity; making a buffer with a given pH value YouTube What Is Higher Buffer Capacity Mean The capacity of a buffer and its initial ph impact the ph change after the addition of an. Buffer capacity decreases significantly when the ph is. A buffer has its highest capacity when the ph is equal to the pka of its acidic component. On the other hand, buffer. Buffer solutions have a working ph range and capacity which dictate. What Is Higher Buffer Capacity Mean.
From www.slideserve.com
PPT Determination of Buffer Capacity PowerPoint Presentation, free What Is Higher Buffer Capacity Mean If you remember high school chemistry or took a college course like chemistry 101, you will have conducted a titration test. Buffer solutions have a working ph range and capacity which dictate how much acid/base can be neutralized before ph changes, and the amount by. Buffer capacity decreases significantly when the ph is. A buffer has its highest capacity when. What Is Higher Buffer Capacity Mean.
From www.slideserve.com
PPT Buffering Capacity Addition of STRONG Acids or Bases PowerPoint What Is Higher Buffer Capacity Mean Buffer capacity refers to the ability of a buffer solution to resist changes in its ph when an acid or base is added. On the other hand, buffer. A buffer has its highest capacity when the ph is equal to the pka of its acidic component. Buffers are characterized by the ph range over which they can maintain a more. What Is Higher Buffer Capacity Mean.
From study.com
Identifying Buffer Capacity Chemistry What Is Higher Buffer Capacity Mean The capacity of a buffer and its initial ph impact the ph change after the addition of an. The buffer capacity is a measure of resistant a particular solution is resistant to change in ph when an acid or a base is added to it. Buffers are characterized by the ph range over which they can maintain a more or. What Is Higher Buffer Capacity Mean.
From www.youtube.com
Buffer Capacity YouTube What Is Higher Buffer Capacity Mean Buffer capacity refers to the ability of a buffer solution to resist changes in its ph when an acid or base is added. Buffer capacity decreases significantly when the ph is. If you remember high school chemistry or took a college course like chemistry 101, you will have conducted a titration test. A buffer has its highest capacity when the. What Is Higher Buffer Capacity Mean.
From www.slideserve.com
PPT Chapter 15 PowerPoint Presentation, free download ID1299205 What Is Higher Buffer Capacity Mean Buffers are characterized by the ph range over which they can maintain a more or less constant ph and by their buffer capacity, the amount of strong acid or base that can be absorbed before the ph changes significantly. Buffer capacity decreases significantly when the ph is. Buffer capacity refers to the ability of a buffer solution to resist changes. What Is Higher Buffer Capacity Mean.
From www.slideserve.com
PPT Buffer Capacity Lab PowerPoint Presentation, free download ID What Is Higher Buffer Capacity Mean Buffer capacity refers to the ability of a buffer solution to resist changes in its ph when an acid or base is added. On the other hand, buffer. Buffers are characterized by the ph range over which they can maintain a more or less constant ph and by their buffer capacity, the amount of strong acid or base that can. What Is Higher Buffer Capacity Mean.
From www.studypool.com
SOLUTION 4 buffering capacity Studypool What Is Higher Buffer Capacity Mean A buffer has its highest capacity when the ph is equal to the pka of its acidic component. Buffer capacity decreases significantly when the ph is. Buffer solutions have a working ph range and capacity which dictate how much acid/base can be neutralized before ph changes, and the amount by. On the other hand, buffer. Buffer capacity refers to the. What Is Higher Buffer Capacity Mean.
From www.slideserve.com
PPT Determination of Buffer Capacity PowerPoint Presentation, free What Is Higher Buffer Capacity Mean On the other hand, buffer. If you remember high school chemistry or took a college course like chemistry 101, you will have conducted a titration test. A buffer has its highest capacity when the ph is equal to the pka of its acidic component. Buffer capacity decreases significantly when the ph is. Buffer solutions have a working ph range and. What Is Higher Buffer Capacity Mean.
From pharmacyscope.com
How do you calculate buffer capacity? Pharmacy Scope What Is Higher Buffer Capacity Mean If you remember high school chemistry or took a college course like chemistry 101, you will have conducted a titration test. The capacity of a buffer and its initial ph impact the ph change after the addition of an. On the other hand, buffer. Buffer solutions have a working ph range and capacity which dictate how much acid/base can be. What Is Higher Buffer Capacity Mean.
From www.slideshare.net
Buffer and Buffer capacity What Is Higher Buffer Capacity Mean Buffers are characterized by the ph range over which they can maintain a more or less constant ph and by their buffer capacity, the amount of strong acid or base that can be absorbed before the ph changes significantly. On the other hand, buffer. The capacity of a buffer and its initial ph impact the ph change after the addition. What Is Higher Buffer Capacity Mean.
From www.slideserve.com
PPT Chapter 15 PowerPoint Presentation, free download ID844461 What Is Higher Buffer Capacity Mean Buffer solutions have a working ph range and capacity which dictate how much acid/base can be neutralized before ph changes, and the amount by. A buffer has its highest capacity when the ph is equal to the pka of its acidic component. Buffer capacity decreases significantly when the ph is. The capacity of a buffer and its initial ph impact. What Is Higher Buffer Capacity Mean.
From www.youtube.com
Buffer range and capacity YouTube What Is Higher Buffer Capacity Mean Buffer capacity decreases significantly when the ph is. Buffer capacity refers to the ability of a buffer solution to resist changes in its ph when an acid or base is added. The capacity of a buffer and its initial ph impact the ph change after the addition of an. On the other hand, buffer. If you remember high school chemistry. What Is Higher Buffer Capacity Mean.
From dxobuticl.blob.core.windows.net
Commonly Used Buffers In The Laboratory at Savannah Osgood blog What Is Higher Buffer Capacity Mean Buffer capacity refers to the ability of a buffer solution to resist changes in its ph when an acid or base is added. The buffer capacity is a measure of resistant a particular solution is resistant to change in ph when an acid or a base is added to it. Buffers are characterized by the ph range over which they. What Is Higher Buffer Capacity Mean.
From en.ppt-online.org
Solutions. Acidbase equilibrium in biological systems online What Is Higher Buffer Capacity Mean Buffer capacity refers to the ability of a buffer solution to resist changes in its ph when an acid or base is added. The buffer capacity is a measure of resistant a particular solution is resistant to change in ph when an acid or a base is added to it. A buffer has its highest capacity when the ph is. What Is Higher Buffer Capacity Mean.
From www.youtube.com
Buffer Range and Buffer Capacity.mp4 YouTube What Is Higher Buffer Capacity Mean The capacity of a buffer and its initial ph impact the ph change after the addition of an. Buffer capacity decreases significantly when the ph is. Buffer solutions have a working ph range and capacity which dictate how much acid/base can be neutralized before ph changes, and the amount by. A buffer has its highest capacity when the ph is. What Is Higher Buffer Capacity Mean.
From www.slideserve.com
PPT Chapter 8 & 9 PowerPoint Presentation, free download ID4339389 What Is Higher Buffer Capacity Mean Buffer capacity decreases significantly when the ph is. Buffer solutions have a working ph range and capacity which dictate how much acid/base can be neutralized before ph changes, and the amount by. The buffer capacity is a measure of resistant a particular solution is resistant to change in ph when an acid or a base is added to it. The. What Is Higher Buffer Capacity Mean.
From www.youtube.com
Buffer capacity Acids and bases AP Chemistry Khan Academy YouTube What Is Higher Buffer Capacity Mean Buffer capacity refers to the ability of a buffer solution to resist changes in its ph when an acid or base is added. If you remember high school chemistry or took a college course like chemistry 101, you will have conducted a titration test. The buffer capacity is a measure of resistant a particular solution is resistant to change in. What Is Higher Buffer Capacity Mean.