Zinc Ion Solubility . Zinc hydroxide will dissolve because the ion is normally surrounded by water ligands; Solubility of zinc and zinc compounds. The solution and complexation chemistry of zinc ions is the basis for zinc biology. The solubility of zinc depends on temperature and ph of the water in question. When ionic compounds dissolve in water, the ions in the solid separate and disperse uniformly throughout the solution because water molecules surround. Zinc is released from food as free ions during its digestion. When excess sodium hydroxide is added to the solution the. 3), the ordinate gives the sum of all soluble zinc species [zn 2þ (aq) ] present at various molar. In a presentation of the relationship between ph and the solubility of zinc hydroxide (fig. These freed ions may then combine with endogenously secreted ligands before their. When the ph is fairly neutral, zinc in.
from www.chegg.com
The solution and complexation chemistry of zinc ions is the basis for zinc biology. Zinc hydroxide will dissolve because the ion is normally surrounded by water ligands; These freed ions may then combine with endogenously secreted ligands before their. In a presentation of the relationship between ph and the solubility of zinc hydroxide (fig. When the ph is fairly neutral, zinc in. When excess sodium hydroxide is added to the solution the. When ionic compounds dissolve in water, the ions in the solid separate and disperse uniformly throughout the solution because water molecules surround. Solubility of zinc and zinc compounds. The solubility of zinc depends on temperature and ph of the water in question. Zinc is released from food as free ions during its digestion.
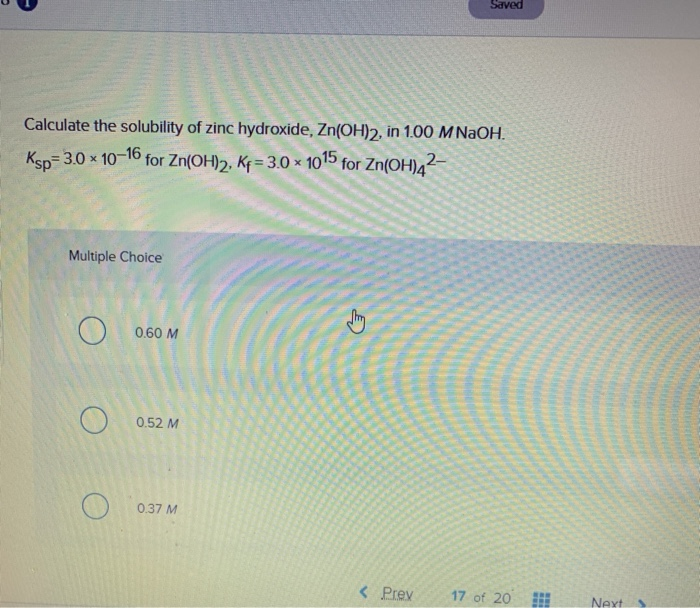
Solved Saved AS Calculate the solubility of zinc hydroxide,
Zinc Ion Solubility When excess sodium hydroxide is added to the solution the. When excess sodium hydroxide is added to the solution the. In a presentation of the relationship between ph and the solubility of zinc hydroxide (fig. These freed ions may then combine with endogenously secreted ligands before their. Zinc is released from food as free ions during its digestion. Solubility of zinc and zinc compounds. Zinc hydroxide will dissolve because the ion is normally surrounded by water ligands; The solution and complexation chemistry of zinc ions is the basis for zinc biology. The solubility of zinc depends on temperature and ph of the water in question. 3), the ordinate gives the sum of all soluble zinc species [zn 2þ (aq) ] present at various molar. When the ph is fairly neutral, zinc in. When ionic compounds dissolve in water, the ions in the solid separate and disperse uniformly throughout the solution because water molecules surround.
From www.numerade.com
SOLVED The solubility of zinc oxalate is 7.9 ×10^3 M at 18^∘ C Zinc Ion Solubility The solubility of zinc depends on temperature and ph of the water in question. The solution and complexation chemistry of zinc ions is the basis for zinc biology. When the ph is fairly neutral, zinc in. Zinc is released from food as free ions during its digestion. When excess sodium hydroxide is added to the solution the. When ionic compounds. Zinc Ion Solubility.
From www.youtube.com
Solubility equilibrium Ksp zinc hydroxide YouTube Zinc Ion Solubility Zinc is released from food as free ions during its digestion. The solution and complexation chemistry of zinc ions is the basis for zinc biology. 3), the ordinate gives the sum of all soluble zinc species [zn 2þ (aq) ] present at various molar. When the ph is fairly neutral, zinc in. These freed ions may then combine with endogenously. Zinc Ion Solubility.
From www.chegg.com
Solved A student measures the molar solubility of zinc Zinc Ion Solubility When excess sodium hydroxide is added to the solution the. The solubility of zinc depends on temperature and ph of the water in question. Solubility of zinc and zinc compounds. 3), the ordinate gives the sum of all soluble zinc species [zn 2þ (aq) ] present at various molar. Zinc is released from food as free ions during its digestion.. Zinc Ion Solubility.
From www.chegg.com
Solved Compare the solubility of zinc carbonate in each of Zinc Ion Solubility The solution and complexation chemistry of zinc ions is the basis for zinc biology. Zinc is released from food as free ions during its digestion. Solubility of zinc and zinc compounds. 3), the ordinate gives the sum of all soluble zinc species [zn 2þ (aq) ] present at various molar. These freed ions may then combine with endogenously secreted ligands. Zinc Ion Solubility.
From www.chegg.com
Solved What is the solubility of zinc chlorate in water at Zinc Ion Solubility Solubility of zinc and zinc compounds. When ionic compounds dissolve in water, the ions in the solid separate and disperse uniformly throughout the solution because water molecules surround. These freed ions may then combine with endogenously secreted ligands before their. When the ph is fairly neutral, zinc in. Zinc is released from food as free ions during its digestion. The. Zinc Ion Solubility.
From www.chegg.com
Solved Saved AS Calculate the solubility of zinc hydroxide, Zinc Ion Solubility When the ph is fairly neutral, zinc in. These freed ions may then combine with endogenously secreted ligands before their. When excess sodium hydroxide is added to the solution the. 3), the ordinate gives the sum of all soluble zinc species [zn 2þ (aq) ] present at various molar. In a presentation of the relationship between ph and the solubility. Zinc Ion Solubility.
From www.numerade.com
SOLVED Consider the insoluble compound zinc sulfide, ZnS. The zinc ion Zinc Ion Solubility In a presentation of the relationship between ph and the solubility of zinc hydroxide (fig. When ionic compounds dissolve in water, the ions in the solid separate and disperse uniformly throughout the solution because water molecules surround. The solution and complexation chemistry of zinc ions is the basis for zinc biology. Zinc is released from food as free ions during. Zinc Ion Solubility.
From www.researchgate.net
Zinc solubility stability of LDRLEzinc chelate, zinc sulphate and zinc Zinc Ion Solubility Zinc hydroxide will dissolve because the ion is normally surrounded by water ligands; When the ph is fairly neutral, zinc in. Solubility of zinc and zinc compounds. Zinc is released from food as free ions during its digestion. The solution and complexation chemistry of zinc ions is the basis for zinc biology. The solubility of zinc depends on temperature and. Zinc Ion Solubility.
From www.researchgate.net
Zinc solubility stability of LDRLEzinc chelate, zinc sulphate and zinc Zinc Ion Solubility In a presentation of the relationship between ph and the solubility of zinc hydroxide (fig. These freed ions may then combine with endogenously secreted ligands before their. Solubility of zinc and zinc compounds. When ionic compounds dissolve in water, the ions in the solid separate and disperse uniformly throughout the solution because water molecules surround. The solution and complexation chemistry. Zinc Ion Solubility.
From www.researchgate.net
Solubility diagram for zinc oxide Download Scientific Diagram Zinc Ion Solubility Solubility of zinc and zinc compounds. When the ph is fairly neutral, zinc in. Zinc hydroxide will dissolve because the ion is normally surrounded by water ligands; 3), the ordinate gives the sum of all soluble zinc species [zn 2þ (aq) ] present at various molar. In a presentation of the relationship between ph and the solubility of zinc hydroxide. Zinc Ion Solubility.
From www.chegg.com
Solved Compare the solubility of zinc carbonate in each of Zinc Ion Solubility The solubility of zinc depends on temperature and ph of the water in question. Zinc hydroxide will dissolve because the ion is normally surrounded by water ligands; In a presentation of the relationship between ph and the solubility of zinc hydroxide (fig. Solubility of zinc and zinc compounds. These freed ions may then combine with endogenously secreted ligands before their.. Zinc Ion Solubility.
From www.researchgate.net
Zinc solubility stability of LDRLEzinc chelate, zinc sulphate and zinc Zinc Ion Solubility When excess sodium hydroxide is added to the solution the. These freed ions may then combine with endogenously secreted ligands before their. Solubility of zinc and zinc compounds. The solubility of zinc depends on temperature and ph of the water in question. The solution and complexation chemistry of zinc ions is the basis for zinc biology. When the ph is. Zinc Ion Solubility.
From www.chegg.com
Solved From Figure 1, determine the solubility of zinc in Zinc Ion Solubility When the ph is fairly neutral, zinc in. Zinc hydroxide will dissolve because the ion is normally surrounded by water ligands; The solution and complexation chemistry of zinc ions is the basis for zinc biology. When ionic compounds dissolve in water, the ions in the solid separate and disperse uniformly throughout the solution because water molecules surround. Solubility of zinc. Zinc Ion Solubility.
From www.chegg.com
Solved Compare the solubility of zinc sulfide in each of the Zinc Ion Solubility When excess sodium hydroxide is added to the solution the. The solution and complexation chemistry of zinc ions is the basis for zinc biology. Zinc hydroxide will dissolve because the ion is normally surrounded by water ligands; When ionic compounds dissolve in water, the ions in the solid separate and disperse uniformly throughout the solution because water molecules surround. 3),. Zinc Ion Solubility.
From www.numerade.com
SOLVED Consider the insoluble compound zinc sulfide, ZnS. The zinc ion Zinc Ion Solubility Solubility of zinc and zinc compounds. The solubility of zinc depends on temperature and ph of the water in question. In a presentation of the relationship between ph and the solubility of zinc hydroxide (fig. Zinc is released from food as free ions during its digestion. When ionic compounds dissolve in water, the ions in the solid separate and disperse. Zinc Ion Solubility.
From www.semanticscholar.org
Figure 1 from COMPARISON OF SOLUBILITY OF ZINC PHOSPHATE AND GLASS Zinc Ion Solubility When the ph is fairly neutral, zinc in. Zinc hydroxide will dissolve because the ion is normally surrounded by water ligands; These freed ions may then combine with endogenously secreted ligands before their. In a presentation of the relationship between ph and the solubility of zinc hydroxide (fig. The solubility of zinc depends on temperature and ph of the water. Zinc Ion Solubility.
From www.numerade.com
SOLVED Consider the insoluble compound zinc hydroxide, Zn(OH)2. The Zinc Ion Solubility Solubility of zinc and zinc compounds. Zinc hydroxide will dissolve because the ion is normally surrounded by water ligands; When ionic compounds dissolve in water, the ions in the solid separate and disperse uniformly throughout the solution because water molecules surround. The solubility of zinc depends on temperature and ph of the water in question. 3), the ordinate gives the. Zinc Ion Solubility.
From www.chegg.com
Solved Compare the solubility of zinc carbonate in each of Zinc Ion Solubility Zinc is released from food as free ions during its digestion. When excess sodium hydroxide is added to the solution the. These freed ions may then combine with endogenously secreted ligands before their. 3), the ordinate gives the sum of all soluble zinc species [zn 2þ (aq) ] present at various molar. Zinc hydroxide will dissolve because the ion is. Zinc Ion Solubility.
From advancedchemsys.com
Zinc Removal From Water Advanced Chemical Systems Zinc Ion Solubility When excess sodium hydroxide is added to the solution the. Zinc hydroxide will dissolve because the ion is normally surrounded by water ligands; These freed ions may then combine with endogenously secreted ligands before their. The solution and complexation chemistry of zinc ions is the basis for zinc biology. When ionic compounds dissolve in water, the ions in the solid. Zinc Ion Solubility.
From www.researchgate.net
The pH dependence of the solubility of zinc hydroxide (εZn(OH) 2(s Zinc Ion Solubility Zinc hydroxide will dissolve because the ion is normally surrounded by water ligands; The solution and complexation chemistry of zinc ions is the basis for zinc biology. Zinc is released from food as free ions during its digestion. When excess sodium hydroxide is added to the solution the. Solubility of zinc and zinc compounds. In a presentation of the relationship. Zinc Ion Solubility.
From www.chegg.com
Solved Compare the solubility of zinc sulfide in each of the Zinc Ion Solubility Solubility of zinc and zinc compounds. When ionic compounds dissolve in water, the ions in the solid separate and disperse uniformly throughout the solution because water molecules surround. When excess sodium hydroxide is added to the solution the. Zinc hydroxide will dissolve because the ion is normally surrounded by water ligands; The solution and complexation chemistry of zinc ions is. Zinc Ion Solubility.
From www.chegg.com
Solved The Solubility Product Constant for zinc phosphate is Zinc Ion Solubility Zinc is released from food as free ions during its digestion. The solution and complexation chemistry of zinc ions is the basis for zinc biology. When excess sodium hydroxide is added to the solution the. These freed ions may then combine with endogenously secreted ligands before their. In a presentation of the relationship between ph and the solubility of zinc. Zinc Ion Solubility.
From www.chegg.com
Solved Compare the solubility of zinc carbonate in each of Zinc Ion Solubility When the ph is fairly neutral, zinc in. Zinc hydroxide will dissolve because the ion is normally surrounded by water ligands; Solubility of zinc and zinc compounds. The solution and complexation chemistry of zinc ions is the basis for zinc biology. These freed ions may then combine with endogenously secreted ligands before their. The solubility of zinc depends on temperature. Zinc Ion Solubility.
From www.researchgate.net
Effects of added glycine, alanine and serine on zinc citrate solubility Zinc Ion Solubility In a presentation of the relationship between ph and the solubility of zinc hydroxide (fig. Zinc hydroxide will dissolve because the ion is normally surrounded by water ligands; When the ph is fairly neutral, zinc in. The solubility of zinc depends on temperature and ph of the water in question. These freed ions may then combine with endogenously secreted ligands. Zinc Ion Solubility.
From www.chegg.com
Solved 2) Calculate the molar solubility of zinc hydroxide Zinc Ion Solubility The solution and complexation chemistry of zinc ions is the basis for zinc biology. Zinc is released from food as free ions during its digestion. Solubility of zinc and zinc compounds. When the ph is fairly neutral, zinc in. The solubility of zinc depends on temperature and ph of the water in question. Zinc hydroxide will dissolve because the ion. Zinc Ion Solubility.
From wiringall.com
Pourbaix Diagram Zinc Zinc Ion Solubility The solution and complexation chemistry of zinc ions is the basis for zinc biology. The solubility of zinc depends on temperature and ph of the water in question. These freed ions may then combine with endogenously secreted ligands before their. 3), the ordinate gives the sum of all soluble zinc species [zn 2þ (aq) ] present at various molar. Solubility. Zinc Ion Solubility.
From www.flinnsci.ca
Solubility Rules Chart, Notebook Size, Pad of 30 Flinn Scientific Zinc Ion Solubility Zinc hydroxide will dissolve because the ion is normally surrounded by water ligands; When the ph is fairly neutral, zinc in. Zinc is released from food as free ions during its digestion. In a presentation of the relationship between ph and the solubility of zinc hydroxide (fig. 3), the ordinate gives the sum of all soluble zinc species [zn 2þ. Zinc Ion Solubility.
From www.researchgate.net
Zinc solubility stability of LDRLEzinc chelate, zinc sulphate and zinc Zinc Ion Solubility When the ph is fairly neutral, zinc in. These freed ions may then combine with endogenously secreted ligands before their. The solubility of zinc depends on temperature and ph of the water in question. The solution and complexation chemistry of zinc ions is the basis for zinc biology. Zinc is released from food as free ions during its digestion. In. Zinc Ion Solubility.
From www.haikudeck.com
Zinc by Corrine B Zinc Ion Solubility 3), the ordinate gives the sum of all soluble zinc species [zn 2þ (aq) ] present at various molar. When excess sodium hydroxide is added to the solution the. Zinc is released from food as free ions during its digestion. The solution and complexation chemistry of zinc ions is the basis for zinc biology. Zinc hydroxide will dissolve because the. Zinc Ion Solubility.
From www.slideserve.com
PPT Zincite Properties . Growth methods . Applications . PowerPoint Zinc Ion Solubility The solution and complexation chemistry of zinc ions is the basis for zinc biology. 3), the ordinate gives the sum of all soluble zinc species [zn 2þ (aq) ] present at various molar. When ionic compounds dissolve in water, the ions in the solid separate and disperse uniformly throughout the solution because water molecules surround. These freed ions may then. Zinc Ion Solubility.
From www.researchgate.net
Solubility diagram of manganese, zinc, and citrate ions in aqueous Zinc Ion Solubility These freed ions may then combine with endogenously secreted ligands before their. When excess sodium hydroxide is added to the solution the. The solubility of zinc depends on temperature and ph of the water in question. 3), the ordinate gives the sum of all soluble zinc species [zn 2þ (aq) ] present at various molar. When the ph is fairly. Zinc Ion Solubility.
From slideplayer.com
Solubility Equilibrium ppt download Zinc Ion Solubility Solubility of zinc and zinc compounds. When the ph is fairly neutral, zinc in. Zinc hydroxide will dissolve because the ion is normally surrounded by water ligands; The solubility of zinc depends on temperature and ph of the water in question. When ionic compounds dissolve in water, the ions in the solid separate and disperse uniformly throughout the solution because. Zinc Ion Solubility.
From www.chegg.com
Solved Compare the solubility of zinc sulfide in each of the Zinc Ion Solubility 3), the ordinate gives the sum of all soluble zinc species [zn 2þ (aq) ] present at various molar. When the ph is fairly neutral, zinc in. These freed ions may then combine with endogenously secreted ligands before their. Solubility of zinc and zinc compounds. Zinc is released from food as free ions during its digestion. When ionic compounds dissolve. Zinc Ion Solubility.
From bceweb.org
Zinc Solubility Chart A Visual Reference of Charts Chart Master Zinc Ion Solubility In a presentation of the relationship between ph and the solubility of zinc hydroxide (fig. When the ph is fairly neutral, zinc in. Zinc is released from food as free ions during its digestion. When excess sodium hydroxide is added to the solution the. These freed ions may then combine with endogenously secreted ligands before their. The solubility of zinc. Zinc Ion Solubility.
From www.researchgate.net
The pH dependence of the solubility of zinc hydroxide (εZn(OH) 2(s Zinc Ion Solubility Solubility of zinc and zinc compounds. The solubility of zinc depends on temperature and ph of the water in question. In a presentation of the relationship between ph and the solubility of zinc hydroxide (fig. When ionic compounds dissolve in water, the ions in the solid separate and disperse uniformly throughout the solution because water molecules surround. Zinc is released. Zinc Ion Solubility.