Non Laboratory Environment . Under the gmp requirements, the manufacturing. Point‐of‐care tests (pocts), which allow for rapid diagnosis of infectious diseases in non‐laboratory settings, have the potential to. The gmp clean air grades and classifications define the environment in which sterile drugs and biological products should be manufactured. He includes tips for staff who want. This blog outlines some of the considerations that go into developing a diagnostic product intended for use outside a traditional laboratory.
from www.expii.com
He includes tips for staff who want. The gmp clean air grades and classifications define the environment in which sterile drugs and biological products should be manufactured. Under the gmp requirements, the manufacturing. Point‐of‐care tests (pocts), which allow for rapid diagnosis of infectious diseases in non‐laboratory settings, have the potential to. This blog outlines some of the considerations that go into developing a diagnostic product intended for use outside a traditional laboratory.
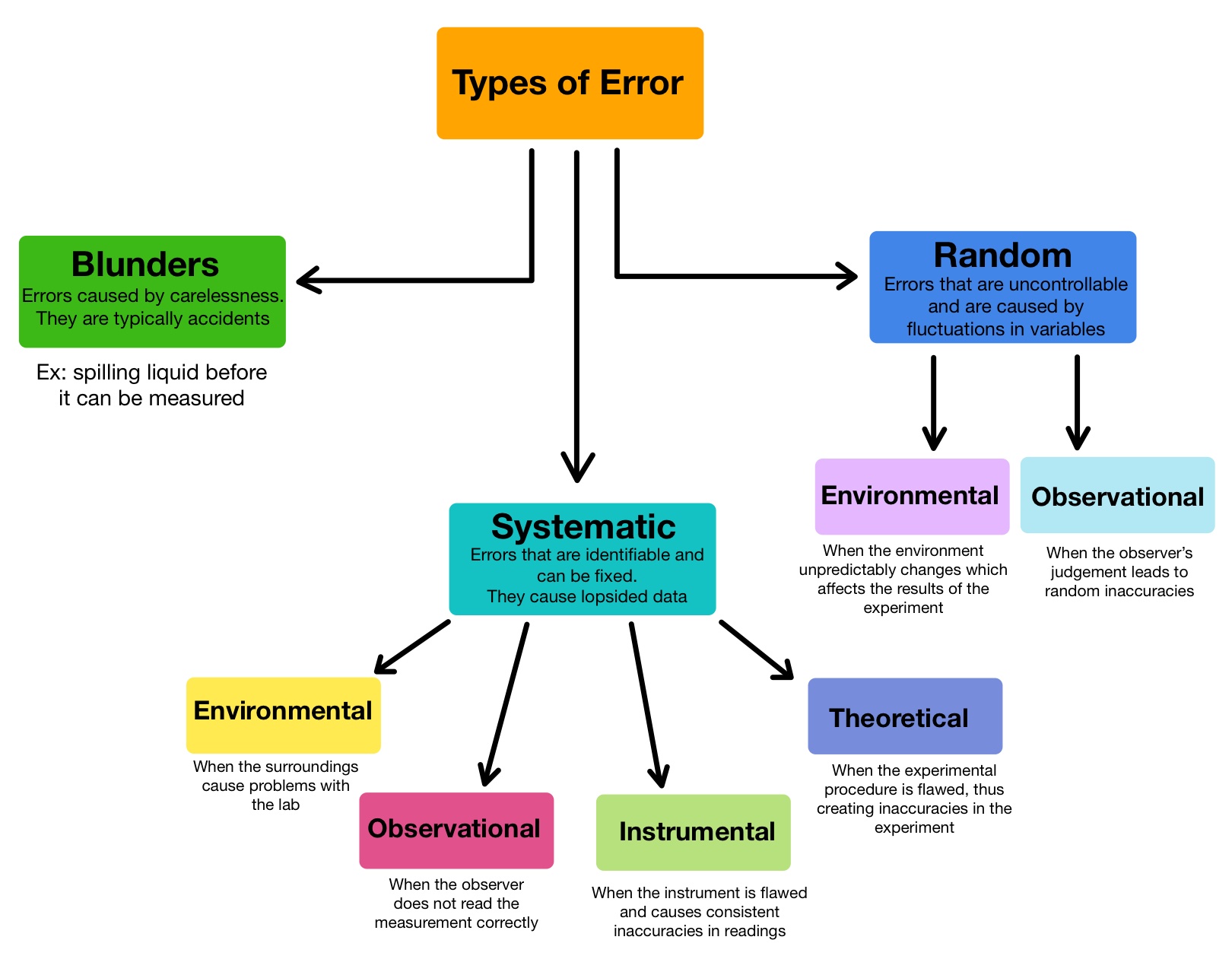
Types of Error — Overview & Comparison Expii
Non Laboratory Environment Under the gmp requirements, the manufacturing. This blog outlines some of the considerations that go into developing a diagnostic product intended for use outside a traditional laboratory. He includes tips for staff who want. Under the gmp requirements, the manufacturing. The gmp clean air grades and classifications define the environment in which sterile drugs and biological products should be manufactured. Point‐of‐care tests (pocts), which allow for rapid diagnosis of infectious diseases in non‐laboratory settings, have the potential to.
From biotix.com
Actions You Can Take Immediately Towards a More EcoFriendly Laboratory Non Laboratory Environment Point‐of‐care tests (pocts), which allow for rapid diagnosis of infectious diseases in non‐laboratory settings, have the potential to. This blog outlines some of the considerations that go into developing a diagnostic product intended for use outside a traditional laboratory. Under the gmp requirements, the manufacturing. The gmp clean air grades and classifications define the environment in which sterile drugs and. Non Laboratory Environment.
From www.frontiersin.org
Frontiers Reaching unreachables Obstacles and successes of microbial Non Laboratory Environment He includes tips for staff who want. This blog outlines some of the considerations that go into developing a diagnostic product intended for use outside a traditional laboratory. The gmp clean air grades and classifications define the environment in which sterile drugs and biological products should be manufactured. Under the gmp requirements, the manufacturing. Point‐of‐care tests (pocts), which allow for. Non Laboratory Environment.
From www.vecteezy.com
360 hdri panorama inside interior of modern research medical laboratory Non Laboratory Environment He includes tips for staff who want. Under the gmp requirements, the manufacturing. Point‐of‐care tests (pocts), which allow for rapid diagnosis of infectious diseases in non‐laboratory settings, have the potential to. The gmp clean air grades and classifications define the environment in which sterile drugs and biological products should be manufactured. This blog outlines some of the considerations that go. Non Laboratory Environment.
From www.ppsthane.com
Environmental Testing Laboratory Perfect Pollucon Services Non Laboratory Environment He includes tips for staff who want. This blog outlines some of the considerations that go into developing a diagnostic product intended for use outside a traditional laboratory. Point‐of‐care tests (pocts), which allow for rapid diagnosis of infectious diseases in non‐laboratory settings, have the potential to. Under the gmp requirements, the manufacturing. The gmp clean air grades and classifications define. Non Laboratory Environment.
From cefzakdq.blob.core.windows.net
Test Zone Lab Contact Number at Minnie Queen blog Non Laboratory Environment This blog outlines some of the considerations that go into developing a diagnostic product intended for use outside a traditional laboratory. Under the gmp requirements, the manufacturing. He includes tips for staff who want. The gmp clean air grades and classifications define the environment in which sterile drugs and biological products should be manufactured. Point‐of‐care tests (pocts), which allow for. Non Laboratory Environment.
From ehs.utoronto.ca
Laboratory Hazardous Waste Management and Disposal Manual Non Laboratory Environment The gmp clean air grades and classifications define the environment in which sterile drugs and biological products should be manufactured. He includes tips for staff who want. Point‐of‐care tests (pocts), which allow for rapid diagnosis of infectious diseases in non‐laboratory settings, have the potential to. This blog outlines some of the considerations that go into developing a diagnostic product intended. Non Laboratory Environment.
From www.kewaunee.in
Wet Lab vs Dry Lab Kewaunee International Group Non Laboratory Environment This blog outlines some of the considerations that go into developing a diagnostic product intended for use outside a traditional laboratory. Under the gmp requirements, the manufacturing. He includes tips for staff who want. Point‐of‐care tests (pocts), which allow for rapid diagnosis of infectious diseases in non‐laboratory settings, have the potential to. The gmp clean air grades and classifications define. Non Laboratory Environment.
From www.eypae.com
Expediting Laboratory Design Within a Changing Environment EYP Non Laboratory Environment Point‐of‐care tests (pocts), which allow for rapid diagnosis of infectious diseases in non‐laboratory settings, have the potential to. Under the gmp requirements, the manufacturing. The gmp clean air grades and classifications define the environment in which sterile drugs and biological products should be manufactured. He includes tips for staff who want. This blog outlines some of the considerations that go. Non Laboratory Environment.
From www.universitylabpartners.org
Wet Lab vs. Dry Lab for Your Life Science Startup Non Laboratory Environment This blog outlines some of the considerations that go into developing a diagnostic product intended for use outside a traditional laboratory. Under the gmp requirements, the manufacturing. Point‐of‐care tests (pocts), which allow for rapid diagnosis of infectious diseases in non‐laboratory settings, have the potential to. He includes tips for staff who want. The gmp clean air grades and classifications define. Non Laboratory Environment.
From www.vectorstock.com
Science Lab Environment Composition Royalty Free Vector Non Laboratory Environment The gmp clean air grades and classifications define the environment in which sterile drugs and biological products should be manufactured. Under the gmp requirements, the manufacturing. This blog outlines some of the considerations that go into developing a diagnostic product intended for use outside a traditional laboratory. He includes tips for staff who want. Point‐of‐care tests (pocts), which allow for. Non Laboratory Environment.
From joivbfirp.blob.core.windows.net
Environmental Laboratory Network Llc at Jonathan Wagar blog Non Laboratory Environment Under the gmp requirements, the manufacturing. He includes tips for staff who want. Point‐of‐care tests (pocts), which allow for rapid diagnosis of infectious diseases in non‐laboratory settings, have the potential to. This blog outlines some of the considerations that go into developing a diagnostic product intended for use outside a traditional laboratory. The gmp clean air grades and classifications define. Non Laboratory Environment.
From ka.mahidol.ac.th
Environmental Engineering Lab Mahidol University Kanchanaburi Campus Non Laboratory Environment He includes tips for staff who want. The gmp clean air grades and classifications define the environment in which sterile drugs and biological products should be manufactured. Point‐of‐care tests (pocts), which allow for rapid diagnosis of infectious diseases in non‐laboratory settings, have the potential to. Under the gmp requirements, the manufacturing. This blog outlines some of the considerations that go. Non Laboratory Environment.
From civ.chuhai.edu.hk
ENVIRONMENTAL LABORATORY Department of Civil Engineering Non Laboratory Environment Point‐of‐care tests (pocts), which allow for rapid diagnosis of infectious diseases in non‐laboratory settings, have the potential to. He includes tips for staff who want. The gmp clean air grades and classifications define the environment in which sterile drugs and biological products should be manufactured. This blog outlines some of the considerations that go into developing a diagnostic product intended. Non Laboratory Environment.
From www.hundure.com
Lab Intelligent Access Control Solution creates an extremely safe Non Laboratory Environment This blog outlines some of the considerations that go into developing a diagnostic product intended for use outside a traditional laboratory. Under the gmp requirements, the manufacturing. Point‐of‐care tests (pocts), which allow for rapid diagnosis of infectious diseases in non‐laboratory settings, have the potential to. The gmp clean air grades and classifications define the environment in which sterile drugs and. Non Laboratory Environment.
From www.bunnyshell.com
What Is a Test Environment and How Do You Make the Most Out of It? Non Laboratory Environment Point‐of‐care tests (pocts), which allow for rapid diagnosis of infectious diseases in non‐laboratory settings, have the potential to. This blog outlines some of the considerations that go into developing a diagnostic product intended for use outside a traditional laboratory. Under the gmp requirements, the manufacturing. He includes tips for staff who want. The gmp clean air grades and classifications define. Non Laboratory Environment.
From centrak.com
Clinical Lab Environmental Monitoring Non Laboratory Environment The gmp clean air grades and classifications define the environment in which sterile drugs and biological products should be manufactured. Under the gmp requirements, the manufacturing. Point‐of‐care tests (pocts), which allow for rapid diagnosis of infectious diseases in non‐laboratory settings, have the potential to. This blog outlines some of the considerations that go into developing a diagnostic product intended for. Non Laboratory Environment.
From biltmoreconstruction.com
Broward County Environmental Monitoring Lab Biltmore Construction Non Laboratory Environment He includes tips for staff who want. The gmp clean air grades and classifications define the environment in which sterile drugs and biological products should be manufactured. Point‐of‐care tests (pocts), which allow for rapid diagnosis of infectious diseases in non‐laboratory settings, have the potential to. This blog outlines some of the considerations that go into developing a diagnostic product intended. Non Laboratory Environment.
From formaspacecontract.com
Laboratories Wet Lab and Tech Lab Furniture Formaspace Contract Non Laboratory Environment Under the gmp requirements, the manufacturing. The gmp clean air grades and classifications define the environment in which sterile drugs and biological products should be manufactured. Point‐of‐care tests (pocts), which allow for rapid diagnosis of infectious diseases in non‐laboratory settings, have the potential to. This blog outlines some of the considerations that go into developing a diagnostic product intended for. Non Laboratory Environment.
From www.arboles.co.uk
6 Energy Efficiency Tips for a Lab Environment Arboles Non Laboratory Environment The gmp clean air grades and classifications define the environment in which sterile drugs and biological products should be manufactured. This blog outlines some of the considerations that go into developing a diagnostic product intended for use outside a traditional laboratory. Point‐of‐care tests (pocts), which allow for rapid diagnosis of infectious diseases in non‐laboratory settings, have the potential to. He. Non Laboratory Environment.
From primaenvlab.com
PRIMA ENVIRONMENT MONITORING & ANALYTICAL LABORATORY Non Laboratory Environment Under the gmp requirements, the manufacturing. He includes tips for staff who want. This blog outlines some of the considerations that go into developing a diagnostic product intended for use outside a traditional laboratory. Point‐of‐care tests (pocts), which allow for rapid diagnosis of infectious diseases in non‐laboratory settings, have the potential to. The gmp clean air grades and classifications define. Non Laboratory Environment.
From www.expii.com
Types of Error — Overview & Comparison Expii Non Laboratory Environment This blog outlines some of the considerations that go into developing a diagnostic product intended for use outside a traditional laboratory. Under the gmp requirements, the manufacturing. He includes tips for staff who want. The gmp clean air grades and classifications define the environment in which sterile drugs and biological products should be manufactured. Point‐of‐care tests (pocts), which allow for. Non Laboratory Environment.
From www.hygitech.co.uk
Sterilization room layout HYGITECH Academy Non Laboratory Environment Under the gmp requirements, the manufacturing. He includes tips for staff who want. The gmp clean air grades and classifications define the environment in which sterile drugs and biological products should be manufactured. Point‐of‐care tests (pocts), which allow for rapid diagnosis of infectious diseases in non‐laboratory settings, have the potential to. This blog outlines some of the considerations that go. Non Laboratory Environment.
From info.dicksondata.com
Environmental Monitoring How to Maintain Compliance Dickson Non Laboratory Environment He includes tips for staff who want. The gmp clean air grades and classifications define the environment in which sterile drugs and biological products should be manufactured. This blog outlines some of the considerations that go into developing a diagnostic product intended for use outside a traditional laboratory. Point‐of‐care tests (pocts), which allow for rapid diagnosis of infectious diseases in. Non Laboratory Environment.
From www.christianschoolproducts.com
The Best of Science Lab Design Christian School Products Non Laboratory Environment Under the gmp requirements, the manufacturing. Point‐of‐care tests (pocts), which allow for rapid diagnosis of infectious diseases in non‐laboratory settings, have the potential to. This blog outlines some of the considerations that go into developing a diagnostic product intended for use outside a traditional laboratory. He includes tips for staff who want. The gmp clean air grades and classifications define. Non Laboratory Environment.
From www.exeter.ac.uk
Sustainable labs Sustainable labs University of Exeter Non Laboratory Environment Under the gmp requirements, the manufacturing. This blog outlines some of the considerations that go into developing a diagnostic product intended for use outside a traditional laboratory. The gmp clean air grades and classifications define the environment in which sterile drugs and biological products should be manufactured. He includes tips for staff who want. Point‐of‐care tests (pocts), which allow for. Non Laboratory Environment.
From www.suburbantestinglabs.com
Environmental Testing Services Suburban Testing Labs Non Laboratory Environment This blog outlines some of the considerations that go into developing a diagnostic product intended for use outside a traditional laboratory. He includes tips for staff who want. The gmp clean air grades and classifications define the environment in which sterile drugs and biological products should be manufactured. Under the gmp requirements, the manufacturing. Point‐of‐care tests (pocts), which allow for. Non Laboratory Environment.
From ehs.stanford.edu
Laboratory Chemical Waste Guidelines Stanford Environmental Health Non Laboratory Environment Point‐of‐care tests (pocts), which allow for rapid diagnosis of infectious diseases in non‐laboratory settings, have the potential to. Under the gmp requirements, the manufacturing. He includes tips for staff who want. The gmp clean air grades and classifications define the environment in which sterile drugs and biological products should be manufactured. This blog outlines some of the considerations that go. Non Laboratory Environment.
From www.dixonpilot.com
Tips for Creating a Safe Laboratory Environment The Dixon Pilot Non Laboratory Environment The gmp clean air grades and classifications define the environment in which sterile drugs and biological products should be manufactured. This blog outlines some of the considerations that go into developing a diagnostic product intended for use outside a traditional laboratory. Under the gmp requirements, the manufacturing. He includes tips for staff who want. Point‐of‐care tests (pocts), which allow for. Non Laboratory Environment.
From www.crbgroup.com
5 considerations for laboratory site selection CRB Non Laboratory Environment He includes tips for staff who want. Under the gmp requirements, the manufacturing. Point‐of‐care tests (pocts), which allow for rapid diagnosis of infectious diseases in non‐laboratory settings, have the potential to. This blog outlines some of the considerations that go into developing a diagnostic product intended for use outside a traditional laboratory. The gmp clean air grades and classifications define. Non Laboratory Environment.
From www.lambdatest.com
What Is Test Environment? A Complete Guide LambdaTest Non Laboratory Environment He includes tips for staff who want. Under the gmp requirements, the manufacturing. The gmp clean air grades and classifications define the environment in which sterile drugs and biological products should be manufactured. Point‐of‐care tests (pocts), which allow for rapid diagnosis of infectious diseases in non‐laboratory settings, have the potential to. This blog outlines some of the considerations that go. Non Laboratory Environment.
From e360.yale.edu
From Lab to Market BioBased Products Are Gaining Momentum Yale E360 Non Laboratory Environment Point‐of‐care tests (pocts), which allow for rapid diagnosis of infectious diseases in non‐laboratory settings, have the potential to. This blog outlines some of the considerations that go into developing a diagnostic product intended for use outside a traditional laboratory. Under the gmp requirements, the manufacturing. The gmp clean air grades and classifications define the environment in which sterile drugs and. Non Laboratory Environment.
From seacrestservicesca.com
Cleanroom Cleaning Facility Services Sea Crest Facility Services Non Laboratory Environment Point‐of‐care tests (pocts), which allow for rapid diagnosis of infectious diseases in non‐laboratory settings, have the potential to. He includes tips for staff who want. The gmp clean air grades and classifications define the environment in which sterile drugs and biological products should be manufactured. Under the gmp requirements, the manufacturing. This blog outlines some of the considerations that go. Non Laboratory Environment.
From guides.library.yale.edu
Home Conservation & Exhibition Strategies Yale University Library Non Laboratory Environment This blog outlines some of the considerations that go into developing a diagnostic product intended for use outside a traditional laboratory. Point‐of‐care tests (pocts), which allow for rapid diagnosis of infectious diseases in non‐laboratory settings, have the potential to. He includes tips for staff who want. Under the gmp requirements, the manufacturing. The gmp clean air grades and classifications define. Non Laboratory Environment.
From www.chitkara.edu.in
Exploring the Opportunities in B.Sc. Medical Laboratory Science Non Laboratory Environment He includes tips for staff who want. This blog outlines some of the considerations that go into developing a diagnostic product intended for use outside a traditional laboratory. The gmp clean air grades and classifications define the environment in which sterile drugs and biological products should be manufactured. Under the gmp requirements, the manufacturing. Point‐of‐care tests (pocts), which allow for. Non Laboratory Environment.
From www.freepik.com
Premium Vector Lab Safety Protocols vector poster Non Laboratory Environment The gmp clean air grades and classifications define the environment in which sterile drugs and biological products should be manufactured. Under the gmp requirements, the manufacturing. Point‐of‐care tests (pocts), which allow for rapid diagnosis of infectious diseases in non‐laboratory settings, have the potential to. He includes tips for staff who want. This blog outlines some of the considerations that go. Non Laboratory Environment.