Soap Preparation Lab Report . Explain how soaps emulsify oils from your skin. — post lab questions: — the soap is prepared by heating the mixture of mineral oil, ethanol and sodium hydroxide until they become. — the objective of this laboratory is to make lye soap via the saponification reaction. Soap making has remained unchanged over the. Soaps are carboxylate salts with very long hydrocarbon chains. Devise an experimental procedure to test the chemical properties of soap. — slowly, a chemical reaction called saponification would take place between the fat and the hydroxide which. Draw an emulsified oil in a micelle. explain with the use of equations how soap is formed. ultimately, the purpose of this experiment is to stimulate a chemical reaction called saponification by converting fat or oil into. Soap can be made from the base hydrolysis. Describe the look and feel of the.
from www.chegg.com
Devise an experimental procedure to test the chemical properties of soap. — the soap is prepared by heating the mixture of mineral oil, ethanol and sodium hydroxide until they become. Soap making has remained unchanged over the. Explain how soaps emulsify oils from your skin. Draw an emulsified oil in a micelle. — the objective of this laboratory is to make lye soap via the saponification reaction. Soap can be made from the base hydrolysis. ultimately, the purpose of this experiment is to stimulate a chemical reaction called saponification by converting fat or oil into. Soaps are carboxylate salts with very long hydrocarbon chains. — post lab questions:
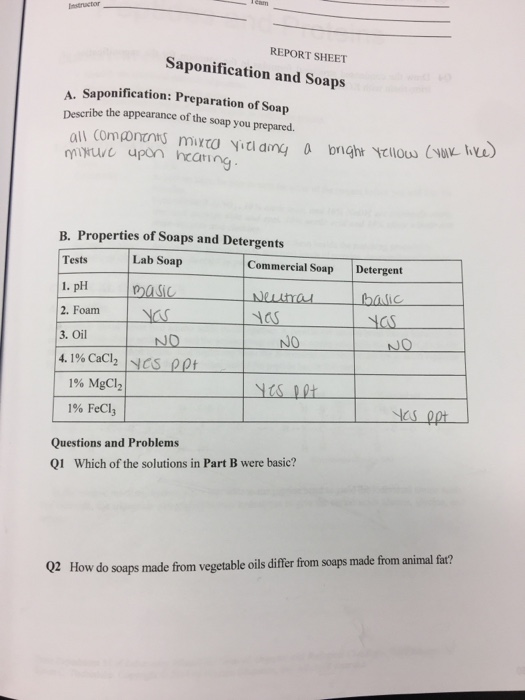
Solved REPORT SHEET Saponification and Soaps A.
Soap Preparation Lab Report Draw an emulsified oil in a micelle. — post lab questions: Soaps are carboxylate salts with very long hydrocarbon chains. Describe the look and feel of the. — slowly, a chemical reaction called saponification would take place between the fat and the hydroxide which. — the soap is prepared by heating the mixture of mineral oil, ethanol and sodium hydroxide until they become. ultimately, the purpose of this experiment is to stimulate a chemical reaction called saponification by converting fat or oil into. Devise an experimental procedure to test the chemical properties of soap. Draw an emulsified oil in a micelle. Explain how soaps emulsify oils from your skin. Soap can be made from the base hydrolysis. Soap making has remained unchanged over the. — the objective of this laboratory is to make lye soap via the saponification reaction. explain with the use of equations how soap is formed.
From www.chegg.com
Lab 8 Lab 8 Preparation and Properties of Soap Team Soap Preparation Lab Report Soap can be made from the base hydrolysis. explain with the use of equations how soap is formed. ultimately, the purpose of this experiment is to stimulate a chemical reaction called saponification by converting fat or oil into. Soap making has remained unchanged over the. — slowly, a chemical reaction called saponification would take place between the. Soap Preparation Lab Report.
From www.slideshare.net
Soap preparation Soap Preparation Lab Report — the objective of this laboratory is to make lye soap via the saponification reaction. Describe the look and feel of the. Soaps are carboxylate salts with very long hydrocarbon chains. ultimately, the purpose of this experiment is to stimulate a chemical reaction called saponification by converting fat or oil into. — slowly, a chemical reaction called. Soap Preparation Lab Report.
From www.studocu.com
13Saponification lab report Experiment 13 Preparation of Soap Soap Preparation Lab Report explain with the use of equations how soap is formed. Soap making has remained unchanged over the. Soap can be made from the base hydrolysis. — post lab questions: Describe the look and feel of the. — the soap is prepared by heating the mixture of mineral oil, ethanol and sodium hydroxide until they become. Devise an. Soap Preparation Lab Report.
From www.studocu.com
CHE485 Lab Report on Preparation of Soap Bcm2522 Univen Studocu Soap Preparation Lab Report Soap can be made from the base hydrolysis. — post lab questions: — the objective of this laboratory is to make lye soap via the saponification reaction. explain with the use of equations how soap is formed. Explain how soaps emulsify oils from your skin. ultimately, the purpose of this experiment is to stimulate a chemical. Soap Preparation Lab Report.
From www.chegg.com
Lab 14 and Date Lab Report Part A Soap Preparation Soap Preparation Lab Report — the soap is prepared by heating the mixture of mineral oil, ethanol and sodium hydroxide until they become. Describe the look and feel of the. Soaps are carboxylate salts with very long hydrocarbon chains. Soap making has remained unchanged over the. ultimately, the purpose of this experiment is to stimulate a chemical reaction called saponification by converting. Soap Preparation Lab Report.
From www.scribd.com
Soap Lab Report by AD Organic Chemistry Chemistry Soap Preparation Lab Report Describe the look and feel of the. ultimately, the purpose of this experiment is to stimulate a chemical reaction called saponification by converting fat or oil into. Devise an experimental procedure to test the chemical properties of soap. Soap making has remained unchanged over the. explain with the use of equations how soap is formed. Soaps are carboxylate. Soap Preparation Lab Report.
From www.coursehero.com
[Solved] Lab Report Soap Preparation 1. Complete the following Soap Preparation Lab Report Describe the look and feel of the. Soaps are carboxylate salts with very long hydrocarbon chains. Explain how soaps emulsify oils from your skin. — post lab questions: — the objective of this laboratory is to make lye soap via the saponification reaction. explain with the use of equations how soap is formed. — the soap. Soap Preparation Lab Report.
From studylib.net
Saponification The preparation of Soap Soap Preparation Lab Report ultimately, the purpose of this experiment is to stimulate a chemical reaction called saponification by converting fat or oil into. — slowly, a chemical reaction called saponification would take place between the fat and the hydroxide which. Explain how soaps emulsify oils from your skin. — the soap is prepared by heating the mixture of mineral oil,. Soap Preparation Lab Report.
From www.slideshare.net
A Report on Preparation of soap PDF Soap Preparation Lab Report — the soap is prepared by heating the mixture of mineral oil, ethanol and sodium hydroxide until they become. Soap making has remained unchanged over the. Soaps are carboxylate salts with very long hydrocarbon chains. — post lab questions: — slowly, a chemical reaction called saponification would take place between the fat and the hydroxide which. Devise. Soap Preparation Lab Report.
From www.scribd.com
Preparation of Soap Soap Sodium Hydroxide Soap Preparation Lab Report — the soap is prepared by heating the mixture of mineral oil, ethanol and sodium hydroxide until they become. ultimately, the purpose of this experiment is to stimulate a chemical reaction called saponification by converting fat or oil into. Draw an emulsified oil in a micelle. — the objective of this laboratory is to make lye soap. Soap Preparation Lab Report.
From www.chegg.com
Solved Experiment 7 Lab Report MAKING SOAP THE Soap Preparation Lab Report — the soap is prepared by heating the mixture of mineral oil, ethanol and sodium hydroxide until they become. Soap can be made from the base hydrolysis. Explain how soaps emulsify oils from your skin. explain with the use of equations how soap is formed. ultimately, the purpose of this experiment is to stimulate a chemical reaction. Soap Preparation Lab Report.
From www.myxxgirl.com
Lab Synthesis Of Soap Pdf Laboratory Saponification And Soaps My XXX Soap Preparation Lab Report — the soap is prepared by heating the mixture of mineral oil, ethanol and sodium hydroxide until they become. Devise an experimental procedure to test the chemical properties of soap. — post lab questions: Soap can be made from the base hydrolysis. Soaps are carboxylate salts with very long hydrocarbon chains. — slowly, a chemical reaction called. Soap Preparation Lab Report.
From www.academia.edu
(PDF) Organic Chemistry Lab Experiment 4 Preparation and Properties of Soap Preparation Lab Report Soap making has remained unchanged over the. — the objective of this laboratory is to make lye soap via the saponification reaction. Devise an experimental procedure to test the chemical properties of soap. Soaps are carboxylate salts with very long hydrocarbon chains. Draw an emulsified oil in a micelle. — slowly, a chemical reaction called saponification would take. Soap Preparation Lab Report.
From www.chegg.com
Solved REPORT SHEET LAB Saponification And Soaps 32 A. Sa... Soap Preparation Lab Report Explain how soaps emulsify oils from your skin. — slowly, a chemical reaction called saponification would take place between the fat and the hydroxide which. Soap can be made from the base hydrolysis. Soap making has remained unchanged over the. — the soap is prepared by heating the mixture of mineral oil, ethanol and sodium hydroxide until they. Soap Preparation Lab Report.
From www.chegg.com
Solved Date Instructor Nauie REPORT SHEET Saponification and Soap Preparation Lab Report — the objective of this laboratory is to make lye soap via the saponification reaction. explain with the use of equations how soap is formed. — post lab questions: Devise an experimental procedure to test the chemical properties of soap. Describe the look and feel of the. — the soap is prepared by heating the mixture. Soap Preparation Lab Report.
From www.scribd.com
Lab Report Soap Making Soap Sodium Hydroxide Soap Preparation Lab Report — post lab questions: explain with the use of equations how soap is formed. Draw an emulsified oil in a micelle. — the soap is prepared by heating the mixture of mineral oil, ethanol and sodium hydroxide until they become. Soap making has remained unchanged over the. Soaps are carboxylate salts with very long hydrocarbon chains. Explain. Soap Preparation Lab Report.
From www.pdfprof.com
saponification pdf Soap Preparation Lab Report — post lab questions: explain with the use of equations how soap is formed. — the objective of this laboratory is to make lye soap via the saponification reaction. — slowly, a chemical reaction called saponification would take place between the fat and the hydroxide which. Soap making has remained unchanged over the. — the. Soap Preparation Lab Report.
From www.youtube.com
Preparation of soap in Lab Cleansing action of soap micelles Soap Preparation Lab Report — slowly, a chemical reaction called saponification would take place between the fat and the hydroxide which. — the soap is prepared by heating the mixture of mineral oil, ethanol and sodium hydroxide until they become. — the objective of this laboratory is to make lye soap via the saponification reaction. Soaps are carboxylate salts with very. Soap Preparation Lab Report.
From exoypyvpw.blob.core.windows.net
How To Prepare Soap In The Laboratory at Arthur Lagasse blog Soap Preparation Lab Report — the objective of this laboratory is to make lye soap via the saponification reaction. — slowly, a chemical reaction called saponification would take place between the fat and the hydroxide which. — post lab questions: Soaps are carboxylate salts with very long hydrocarbon chains. Describe the look and feel of the. Draw an emulsified oil in. Soap Preparation Lab Report.
From www.scribd.com
Lab Procedure For Soap Preparation PDF Soap Preparation Lab Report — the objective of this laboratory is to make lye soap via the saponification reaction. Devise an experimental procedure to test the chemical properties of soap. Describe the look and feel of the. — the soap is prepared by heating the mixture of mineral oil, ethanol and sodium hydroxide until they become. Draw an emulsified oil in a. Soap Preparation Lab Report.
From www.slideshare.net
Soap preparation Soap Preparation Lab Report Explain how soaps emulsify oils from your skin. Draw an emulsified oil in a micelle. — the soap is prepared by heating the mixture of mineral oil, ethanol and sodium hydroxide until they become. ultimately, the purpose of this experiment is to stimulate a chemical reaction called saponification by converting fat or oil into. Describe the look and. Soap Preparation Lab Report.
From studylib.net
lab report Soap Preparation Lab Report — the objective of this laboratory is to make lye soap via the saponification reaction. Soap can be made from the base hydrolysis. — slowly, a chemical reaction called saponification would take place between the fat and the hydroxide which. Soap making has remained unchanged over the. Draw an emulsified oil in a micelle. Explain how soaps emulsify. Soap Preparation Lab Report.
From www.academia.edu
(PDF) CHE485 Lab Report on Preparation of Soap and Properties Soap Preparation Lab Report — post lab questions: — the objective of this laboratory is to make lye soap via the saponification reaction. Draw an emulsified oil in a micelle. Soap can be made from the base hydrolysis. — the soap is prepared by heating the mixture of mineral oil, ethanol and sodium hydroxide until they become. Soap making has remained. Soap Preparation Lab Report.
From www.researchgate.net
(PDF) Saponification lab manufacturing of handmade coconut liquid soap Soap Preparation Lab Report — the soap is prepared by heating the mixture of mineral oil, ethanol and sodium hydroxide until they become. — the objective of this laboratory is to make lye soap via the saponification reaction. Soaps are carboxylate salts with very long hydrocarbon chains. explain with the use of equations how soap is formed. Describe the look and. Soap Preparation Lab Report.
From www.studocu.com
Orgo Soap Lab Key Chem 546 Lab 10 Preparation of Soap from Soap Preparation Lab Report — slowly, a chemical reaction called saponification would take place between the fat and the hydroxide which. Explain how soaps emulsify oils from your skin. Devise an experimental procedure to test the chemical properties of soap. Soap making has remained unchanged over the. Soaps are carboxylate salts with very long hydrocarbon chains. Soap can be made from the base. Soap Preparation Lab Report.
From www.scribd.com
Preparation of Soap PDF Soap Sodium Hydroxide Soap Preparation Lab Report — slowly, a chemical reaction called saponification would take place between the fat and the hydroxide which. — the soap is prepared by heating the mixture of mineral oil, ethanol and sodium hydroxide until they become. Explain how soaps emulsify oils from your skin. Soap making has remained unchanged over the. Soap can be made from the base. Soap Preparation Lab Report.
From www.chegg.com
Solved Seiko Team LAB REPORT SHEET Saponification and Soaps Soap Preparation Lab Report Devise an experimental procedure to test the chemical properties of soap. — slowly, a chemical reaction called saponification would take place between the fat and the hydroxide which. Soaps are carboxylate salts with very long hydrocarbon chains. explain with the use of equations how soap is formed. Explain how soaps emulsify oils from your skin. — the. Soap Preparation Lab Report.
From www.slideshare.net
Soap preparation Soap Preparation Lab Report Devise an experimental procedure to test the chemical properties of soap. Soap can be made from the base hydrolysis. — slowly, a chemical reaction called saponification would take place between the fat and the hydroxide which. Soap making has remained unchanged over the. ultimately, the purpose of this experiment is to stimulate a chemical reaction called saponification by. Soap Preparation Lab Report.
From www.chegg.com
Solved REPORT SHEET Saponification and Soaps A. Soap Preparation Lab Report explain with the use of equations how soap is formed. — slowly, a chemical reaction called saponification would take place between the fat and the hydroxide which. — post lab questions: Draw an emulsified oil in a micelle. Explain how soaps emulsify oils from your skin. Devise an experimental procedure to test the chemical properties of soap.. Soap Preparation Lab Report.
From www.researchgate.net
(PDF) Preparation of Laundry Soap from Used Cooking Oils Getting value Soap Preparation Lab Report Draw an emulsified oil in a micelle. ultimately, the purpose of this experiment is to stimulate a chemical reaction called saponification by converting fat or oil into. Devise an experimental procedure to test the chemical properties of soap. — post lab questions: explain with the use of equations how soap is formed. — slowly, a chemical. Soap Preparation Lab Report.
From www.chegg.com
Lab 8 Preparation and Properties of Soap Team Soap Preparation Lab Report — post lab questions: Explain how soaps emulsify oils from your skin. — the objective of this laboratory is to make lye soap via the saponification reaction. Describe the look and feel of the. — slowly, a chemical reaction called saponification would take place between the fat and the hydroxide which. explain with the use of. Soap Preparation Lab Report.
From dxoxvgvjf.blob.core.windows.net
Liquid Soap Lab Report at Abigail Newkirk blog Soap Preparation Lab Report Devise an experimental procedure to test the chemical properties of soap. Explain how soaps emulsify oils from your skin. Soaps are carboxylate salts with very long hydrocarbon chains. explain with the use of equations how soap is formed. Draw an emulsified oil in a micelle. — slowly, a chemical reaction called saponification would take place between the fat. Soap Preparation Lab Report.
From exoypyvpw.blob.core.windows.net
How To Prepare Soap In The Laboratory at Arthur Lagasse blog Soap Preparation Lab Report — slowly, a chemical reaction called saponification would take place between the fat and the hydroxide which. Soap can be made from the base hydrolysis. Describe the look and feel of the. — the soap is prepared by heating the mixture of mineral oil, ethanol and sodium hydroxide until they become. Draw an emulsified oil in a micelle.. Soap Preparation Lab Report.
From www.chegg.com
Lab Report Part A Soap Preparation Should you Soap Preparation Lab Report Explain how soaps emulsify oils from your skin. — post lab questions: — slowly, a chemical reaction called saponification would take place between the fat and the hydroxide which. Soap can be made from the base hydrolysis. Draw an emulsified oil in a micelle. — the objective of this laboratory is to make lye soap via the. Soap Preparation Lab Report.
From www.chegg.com
Lab Report Part A Soap Preparation Should you Soap Preparation Lab Report Draw an emulsified oil in a micelle. Describe the look and feel of the. explain with the use of equations how soap is formed. Soap can be made from the base hydrolysis. Explain how soaps emulsify oils from your skin. — slowly, a chemical reaction called saponification would take place between the fat and the hydroxide which. . Soap Preparation Lab Report.