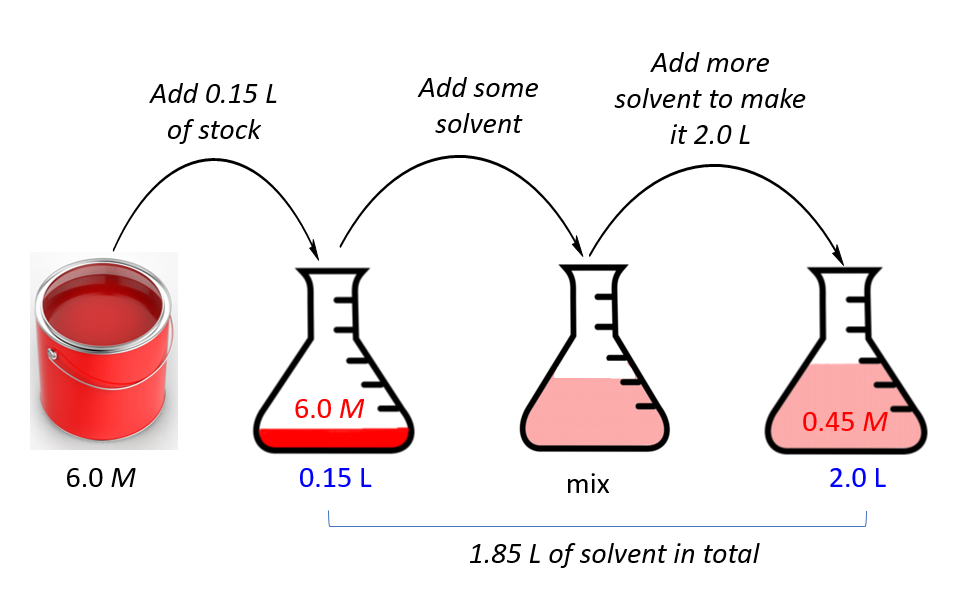
Dilution And Volumes . A dilute solution is one. State whether the concentration of a solution is directly or indirectly proportional to its volume. Calculate the new concentration or volume for a dilution or concentration of a solution. Explanations and examples of common methods. Follow these five steps to make a dilution: Of course, the resulting solution is thoroughly mixed so as to ensure that all parts of the solution are identical. Calculate solution concentrations using molarity. There are many ways of expressing concentration and dilution. To dilute a solution means to add more solvent without the addition of more solute. Describe the fundamental properties of solutions. How to make a dilution. If you do not know them already, use the dilution. We will begin our discussion of solution concentration with two related and relative terms:
from general.chemistrysteps.com
We will begin our discussion of solution concentration with two related and relative terms: State whether the concentration of a solution is directly or indirectly proportional to its volume. Describe the fundamental properties of solutions. Calculate the new concentration or volume for a dilution or concentration of a solution. If you do not know them already, use the dilution. Follow these five steps to make a dilution: How to make a dilution. To dilute a solution means to add more solvent without the addition of more solute. Calculate solution concentrations using molarity. There are many ways of expressing concentration and dilution.
Dilution of a Stock Solution and Calculations Based Morality
Dilution And Volumes A dilute solution is one. We will begin our discussion of solution concentration with two related and relative terms: Calculate the new concentration or volume for a dilution or concentration of a solution. To dilute a solution means to add more solvent without the addition of more solute. Calculate solution concentrations using molarity. Describe the fundamental properties of solutions. State whether the concentration of a solution is directly or indirectly proportional to its volume. Explanations and examples of common methods. A dilute solution is one. If you do not know them already, use the dilution. Follow these five steps to make a dilution: How to make a dilution. Of course, the resulting solution is thoroughly mixed so as to ensure that all parts of the solution are identical. There are many ways of expressing concentration and dilution.
From borenew.weebly.com
Serial Dilution Calculation Examples borenew Dilution And Volumes Calculate the new concentration or volume for a dilution or concentration of a solution. Explanations and examples of common methods. Calculate solution concentrations using molarity. Describe the fundamental properties of solutions. Follow these five steps to make a dilution: To dilute a solution means to add more solvent without the addition of more solute. There are many ways of expressing. Dilution And Volumes.
From www.medicine.mcgill.ca
Serial Dilutions Dilution And Volumes A dilute solution is one. State whether the concentration of a solution is directly or indirectly proportional to its volume. How to make a dilution. Explanations and examples of common methods. Of course, the resulting solution is thoroughly mixed so as to ensure that all parts of the solution are identical. Calculate solution concentrations using molarity. To dilute a solution. Dilution And Volumes.
From www.slideserve.com
PPT Pharmaceutical Calculations (5) PowerPoint Presentation, free Dilution And Volumes State whether the concentration of a solution is directly or indirectly proportional to its volume. We will begin our discussion of solution concentration with two related and relative terms: If you do not know them already, use the dilution. How to make a dilution. Follow these five steps to make a dilution: There are many ways of expressing concentration and. Dilution And Volumes.
From www.slideserve.com
PPT Flow and Volume of Blood PowerPoint Presentation, free download Dilution And Volumes State whether the concentration of a solution is directly or indirectly proportional to its volume. To dilute a solution means to add more solvent without the addition of more solute. A dilute solution is one. We will begin our discussion of solution concentration with two related and relative terms: There are many ways of expressing concentration and dilution. Of course,. Dilution And Volumes.
From sciencequery.com
What is serial dilution method? And how to calculate? Science Query Dilution And Volumes We will begin our discussion of solution concentration with two related and relative terms: A dilute solution is one. Calculate the new concentration or volume for a dilution or concentration of a solution. Calculate solution concentrations using molarity. How to make a dilution. Follow these five steps to make a dilution: If you do not know them already, use the. Dilution And Volumes.
From owlcation.com
Lung Volumes and Capacities Owlcation Dilution And Volumes Calculate solution concentrations using molarity. Follow these five steps to make a dilution: A dilute solution is one. Of course, the resulting solution is thoroughly mixed so as to ensure that all parts of the solution are identical. How to make a dilution. If you do not know them already, use the dilution. Calculate the new concentration or volume for. Dilution And Volumes.
From www.youtube.com
Chemistry 11 Dilution Calculations Solving for Initial Volume Example Dilution And Volumes A dilute solution is one. Of course, the resulting solution is thoroughly mixed so as to ensure that all parts of the solution are identical. We will begin our discussion of solution concentration with two related and relative terms: Follow these five steps to make a dilution: Describe the fundamental properties of solutions. There are many ways of expressing concentration. Dilution And Volumes.
From general.chemistrysteps.com
Dilution of a Stock Solution and Calculations Based Morality Dilution And Volumes Calculate the new concentration or volume for a dilution or concentration of a solution. Of course, the resulting solution is thoroughly mixed so as to ensure that all parts of the solution are identical. Explanations and examples of common methods. How to make a dilution. If you do not know them already, use the dilution. Calculate solution concentrations using molarity.. Dilution And Volumes.
From study.com
Dilution Definition, Equation & Factors Video & Lesson Transcript Dilution And Volumes There are many ways of expressing concentration and dilution. Describe the fundamental properties of solutions. How to make a dilution. Calculate solution concentrations using molarity. If you do not know them already, use the dilution. State whether the concentration of a solution is directly or indirectly proportional to its volume. To dilute a solution means to add more solvent without. Dilution And Volumes.
From www.chegg.com
Solved 3. Given the following dilution scheme, calculate the Dilution And Volumes Calculate solution concentrations using molarity. Of course, the resulting solution is thoroughly mixed so as to ensure that all parts of the solution are identical. Follow these five steps to make a dilution: Calculate the new concentration or volume for a dilution or concentration of a solution. There are many ways of expressing concentration and dilution. Explanations and examples of. Dilution And Volumes.
From www.youtube.com
Dilution calculations Dilution problems Stock dilutions Biology and Dilution And Volumes How to make a dilution. State whether the concentration of a solution is directly or indirectly proportional to its volume. There are many ways of expressing concentration and dilution. Follow these five steps to make a dilution: To dilute a solution means to add more solvent without the addition of more solute. We will begin our discussion of solution concentration. Dilution And Volumes.
From www.youtube.com
Calculating Dilutions C1V1=C2V2 PowerPoint YouTube Dilution And Volumes Calculate the new concentration or volume for a dilution or concentration of a solution. Of course, the resulting solution is thoroughly mixed so as to ensure that all parts of the solution are identical. A dilute solution is one. To dilute a solution means to add more solvent without the addition of more solute. How to make a dilution. There. Dilution And Volumes.
From www.slideshare.net
Molarity and dilution Dilution And Volumes State whether the concentration of a solution is directly or indirectly proportional to its volume. Calculate the new concentration or volume for a dilution or concentration of a solution. How to make a dilution. Follow these five steps to make a dilution: If you do not know them already, use the dilution. Calculate solution concentrations using molarity. We will begin. Dilution And Volumes.
From www.expii.com
Dilution of Solutions — Overview & Examples Expii Dilution And Volumes We will begin our discussion of solution concentration with two related and relative terms: Of course, the resulting solution is thoroughly mixed so as to ensure that all parts of the solution are identical. Follow these five steps to make a dilution: If you do not know them already, use the dilution. Calculate the new concentration or volume for a. Dilution And Volumes.
From www.chegg.com
Solved 2. Bradford Assay. The total dilution volume is 100 Dilution And Volumes A dilute solution is one. Of course, the resulting solution is thoroughly mixed so as to ensure that all parts of the solution are identical. Follow these five steps to make a dilution: We will begin our discussion of solution concentration with two related and relative terms: State whether the concentration of a solution is directly or indirectly proportional to. Dilution And Volumes.
From labpedia.net
Solutions Part 1 Solutions Preparation used in Clinical Laboratory Dilution And Volumes If you do not know them already, use the dilution. Follow these five steps to make a dilution: How to make a dilution. To dilute a solution means to add more solvent without the addition of more solute. Calculate the new concentration or volume for a dilution or concentration of a solution. Of course, the resulting solution is thoroughly mixed. Dilution And Volumes.
From www.madebyteachers.com
Dilution, Molarity, and Volume Calculations A Chemistry Worksheet Dilution And Volumes State whether the concentration of a solution is directly or indirectly proportional to its volume. If you do not know them already, use the dilution. Follow these five steps to make a dilution: Calculate the new concentration or volume for a dilution or concentration of a solution. Describe the fundamental properties of solutions. Calculate solution concentrations using molarity. A dilute. Dilution And Volumes.
From chem370.github.io
Lab 1 Prelab CHEM 370 Dilution And Volumes Describe the fundamental properties of solutions. To dilute a solution means to add more solvent without the addition of more solute. We will begin our discussion of solution concentration with two related and relative terms: There are many ways of expressing concentration and dilution. If you do not know them already, use the dilution. A dilute solution is one. Calculate. Dilution And Volumes.
From www.youtube.com
CHEMISTRY 101 Solution Dilutions YouTube Dilution And Volumes Follow these five steps to make a dilution: Of course, the resulting solution is thoroughly mixed so as to ensure that all parts of the solution are identical. We will begin our discussion of solution concentration with two related and relative terms: Calculate the new concentration or volume for a dilution or concentration of a solution. State whether the concentration. Dilution And Volumes.
From www.youtube.com
Explain Isotopic Dilution. Nuclear Chemistry Physical Chemistry YouTube Dilution And Volumes To dilute a solution means to add more solvent without the addition of more solute. Calculate solution concentrations using molarity. State whether the concentration of a solution is directly or indirectly proportional to its volume. How to make a dilution. Of course, the resulting solution is thoroughly mixed so as to ensure that all parts of the solution are identical.. Dilution And Volumes.
From www.youtube.com
Dilution Chart.Helpful video. Understand how to prepare dilutions in Dilution And Volumes Describe the fundamental properties of solutions. Calculate solution concentrations using molarity. How to make a dilution. Of course, the resulting solution is thoroughly mixed so as to ensure that all parts of the solution are identical. Follow these five steps to make a dilution: A dilute solution is one. We will begin our discussion of solution concentration with two related. Dilution And Volumes.
From www.youtube.com
Dilution and Dilution Factor in Microbiology How to Calculate Dilution And Volumes Calculate the new concentration or volume for a dilution or concentration of a solution. A dilute solution is one. We will begin our discussion of solution concentration with two related and relative terms: There are many ways of expressing concentration and dilution. To dilute a solution means to add more solvent without the addition of more solute. Describe the fundamental. Dilution And Volumes.
From slideplayer.com
Concentration Dilution Solubility ppt download Dilution And Volumes Describe the fundamental properties of solutions. We will begin our discussion of solution concentration with two related and relative terms: Explanations and examples of common methods. Of course, the resulting solution is thoroughly mixed so as to ensure that all parts of the solution are identical. State whether the concentration of a solution is directly or indirectly proportional to its. Dilution And Volumes.
From www.slideshare.net
Molarity and dilution Dilution And Volumes A dilute solution is one. If you do not know them already, use the dilution. How to make a dilution. State whether the concentration of a solution is directly or indirectly proportional to its volume. Describe the fundamental properties of solutions. To dilute a solution means to add more solvent without the addition of more solute. Explanations and examples of. Dilution And Volumes.
From www.youtube.com
Chem 11 Dilution Calculations_Solving for Final Volume after Dilution Dilution And Volumes Explanations and examples of common methods. How to make a dilution. A dilute solution is one. Of course, the resulting solution is thoroughly mixed so as to ensure that all parts of the solution are identical. Calculate solution concentrations using molarity. Follow these five steps to make a dilution: There are many ways of expressing concentration and dilution. State whether. Dilution And Volumes.
From chem.libretexts.org
5.2 Solutions and Dilutions Chemistry LibreTexts Dilution And Volumes There are many ways of expressing concentration and dilution. If you do not know them already, use the dilution. State whether the concentration of a solution is directly or indirectly proportional to its volume. To dilute a solution means to add more solvent without the addition of more solute. Calculate the new concentration or volume for a dilution or concentration. Dilution And Volumes.
From www.slideserve.com
PPT Preparing Solutions with Dilutions PowerPoint Presentation, free Dilution And Volumes Follow these five steps to make a dilution: A dilute solution is one. We will begin our discussion of solution concentration with two related and relative terms: To dilute a solution means to add more solvent without the addition of more solute. Of course, the resulting solution is thoroughly mixed so as to ensure that all parts of the solution. Dilution And Volumes.
From www.youtube.com
Serial Dilution Method Protocol Step Wise Explanation YouTube Dilution And Volumes Explanations and examples of common methods. Calculate solution concentrations using molarity. To dilute a solution means to add more solvent without the addition of more solute. There are many ways of expressing concentration and dilution. If you do not know them already, use the dilution. We will begin our discussion of solution concentration with two related and relative terms: Calculate. Dilution And Volumes.
From www.la-photo-argentique.com
La dilution pour les nuls des chimies argentiques Dilution And Volumes There are many ways of expressing concentration and dilution. We will begin our discussion of solution concentration with two related and relative terms: State whether the concentration of a solution is directly or indirectly proportional to its volume. Follow these five steps to make a dilution: A dilute solution is one. Calculate solution concentrations using molarity. Of course, the resulting. Dilution And Volumes.
From www.madebyteachers.com
Dilution, Molarity, and Volume Calculations A Chemistry Worksheet Dilution And Volumes How to make a dilution. Calculate the new concentration or volume for a dilution or concentration of a solution. If you do not know them already, use the dilution. State whether the concentration of a solution is directly or indirectly proportional to its volume. Of course, the resulting solution is thoroughly mixed so as to ensure that all parts of. Dilution And Volumes.
From chem.libretexts.org
14.7 Solution Dilution Chemistry LibreTexts Dilution And Volumes We will begin our discussion of solution concentration with two related and relative terms: Calculate solution concentrations using molarity. A dilute solution is one. Follow these five steps to make a dilution: Of course, the resulting solution is thoroughly mixed so as to ensure that all parts of the solution are identical. Explanations and examples of common methods. State whether. Dilution And Volumes.
From socratic.org
How can I calculate the dilution factor using concentration? Socratic Dilution And Volumes Calculate solution concentrations using molarity. If you do not know them already, use the dilution. Explanations and examples of common methods. Follow these five steps to make a dilution: Of course, the resulting solution is thoroughly mixed so as to ensure that all parts of the solution are identical. We will begin our discussion of solution concentration with two related. Dilution And Volumes.
From www.chegg.com
Solved The most common dilutions are 1/10, 1/1000 and Dilution And Volumes State whether the concentration of a solution is directly or indirectly proportional to its volume. Describe the fundamental properties of solutions. How to make a dilution. Calculate the new concentration or volume for a dilution or concentration of a solution. Calculate solution concentrations using molarity. To dilute a solution means to add more solvent without the addition of more solute.. Dilution And Volumes.
From www.slideserve.com
PPT Dilution Calculations PowerPoint Presentation, free download ID Dilution And Volumes State whether the concentration of a solution is directly or indirectly proportional to its volume. Describe the fundamental properties of solutions. To dilute a solution means to add more solvent without the addition of more solute. How to make a dilution. Calculate solution concentrations using molarity. Calculate the new concentration or volume for a dilution or concentration of a solution.. Dilution And Volumes.
From twinklsecondary.blog
Products of a Dilution Series A Level Biology Revision Dilution And Volumes If you do not know them already, use the dilution. Calculate solution concentrations using molarity. Explanations and examples of common methods. We will begin our discussion of solution concentration with two related and relative terms: Describe the fundamental properties of solutions. To dilute a solution means to add more solvent without the addition of more solute. A dilute solution is. Dilution And Volumes.