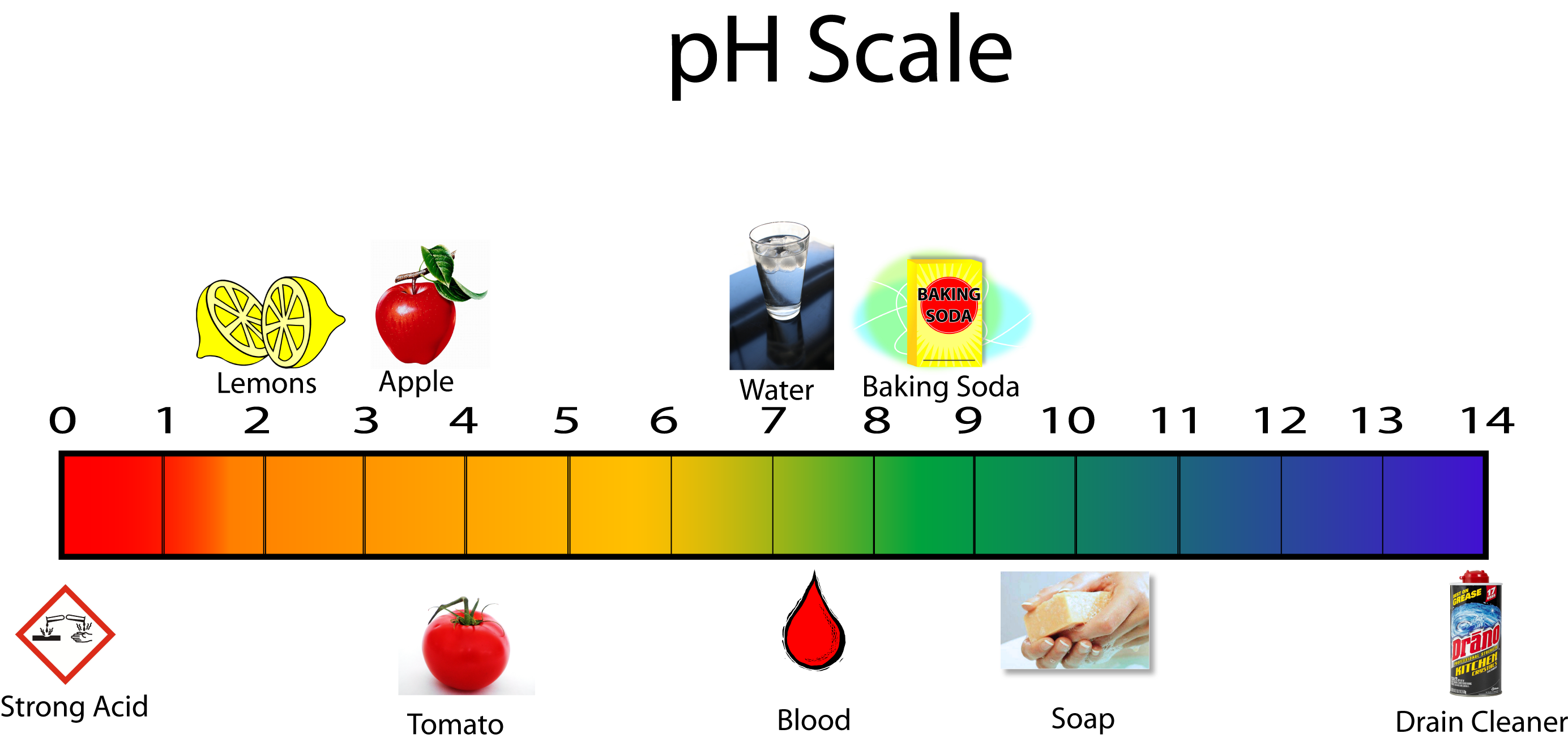
Examples Of Acids Vs Bases . Properties of acids vs bases. the earliest definition of acids and bases is arrhenius's definition which states that: according to the lewis definition, acids are molecules or ions capable of coordinating with unshared electron pairs,. acids react with bases to produce a salt compound and water. throughout history, chemists have created different definitions of acids and bases. When equal moles of an acid and a base are combined, the acid is neutralized by the base. common examples include vinegar (acetic acid, ch₃cooh), citrus fruits (citric acid, c₆h₈o₇), and stomach acid (hydrochloric acid, hcl). Bases have a slippery feel on fingers and taste bitter. discover why acids and bases have a different ph level along with other important properties by looking at each substance. An acid is a substance that forms hydrogen ions h + when.
from mungfali.com
An acid is a substance that forms hydrogen ions h + when. common examples include vinegar (acetic acid, ch₃cooh), citrus fruits (citric acid, c₆h₈o₇), and stomach acid (hydrochloric acid, hcl). acids react with bases to produce a salt compound and water. the earliest definition of acids and bases is arrhenius's definition which states that: throughout history, chemists have created different definitions of acids and bases. according to the lewis definition, acids are molecules or ions capable of coordinating with unshared electron pairs,. discover why acids and bases have a different ph level along with other important properties by looking at each substance. Bases have a slippery feel on fingers and taste bitter. Properties of acids vs bases. When equal moles of an acid and a base are combined, the acid is neutralized by the base.
Acid Base Concept Map
Examples Of Acids Vs Bases common examples include vinegar (acetic acid, ch₃cooh), citrus fruits (citric acid, c₆h₈o₇), and stomach acid (hydrochloric acid, hcl). When equal moles of an acid and a base are combined, the acid is neutralized by the base. according to the lewis definition, acids are molecules or ions capable of coordinating with unshared electron pairs,. throughout history, chemists have created different definitions of acids and bases. discover why acids and bases have a different ph level along with other important properties by looking at each substance. An acid is a substance that forms hydrogen ions h + when. the earliest definition of acids and bases is arrhenius's definition which states that: acids react with bases to produce a salt compound and water. common examples include vinegar (acetic acid, ch₃cooh), citrus fruits (citric acid, c₆h₈o₇), and stomach acid (hydrochloric acid, hcl). Properties of acids vs bases. Bases have a slippery feel on fingers and taste bitter.
From www.chemistrylearner.com
AcidBase Reaction Definition, Examples, and Uses Examples Of Acids Vs Bases acids react with bases to produce a salt compound and water. throughout history, chemists have created different definitions of acids and bases. according to the lewis definition, acids are molecules or ions capable of coordinating with unshared electron pairs,. An acid is a substance that forms hydrogen ions h + when. the earliest definition of acids. Examples Of Acids Vs Bases.
From kidspressmagazine.com
Acids and Bases Examples Of Acids Vs Bases acids react with bases to produce a salt compound and water. When equal moles of an acid and a base are combined, the acid is neutralized by the base. according to the lewis definition, acids are molecules or ions capable of coordinating with unshared electron pairs,. the earliest definition of acids and bases is arrhenius's definition which. Examples Of Acids Vs Bases.
From www.haikudeck.com
Acids Vs. Bases by autumnscor Examples Of Acids Vs Bases discover why acids and bases have a different ph level along with other important properties by looking at each substance. An acid is a substance that forms hydrogen ions h + when. common examples include vinegar (acetic acid, ch₃cooh), citrus fruits (citric acid, c₆h₈o₇), and stomach acid (hydrochloric acid, hcl). Properties of acids vs bases. throughout history,. Examples Of Acids Vs Bases.
From examples.yourdictionary.com
Difference Between Acids and Bases Key Properties Examples Of Acids Vs Bases An acid is a substance that forms hydrogen ions h + when. throughout history, chemists have created different definitions of acids and bases. the earliest definition of acids and bases is arrhenius's definition which states that: according to the lewis definition, acids are molecules or ions capable of coordinating with unshared electron pairs,. discover why acids. Examples Of Acids Vs Bases.
From sciencenotes.org
AcidBase Chemistry Examples Of Acids Vs Bases Bases have a slippery feel on fingers and taste bitter. An acid is a substance that forms hydrogen ions h + when. Properties of acids vs bases. acids react with bases to produce a salt compound and water. according to the lewis definition, acids are molecules or ions capable of coordinating with unshared electron pairs,. throughout history,. Examples Of Acids Vs Bases.
From chemistry-examples-00.blogspot.com
70 CHEMISTRY EXAMPLES OF ACIDS AND BASES, BASES ACIDS EXAMPLES OF CHEMISTRY AND Chemistry Examples Examples Of Acids Vs Bases An acid is a substance that forms hydrogen ions h + when. discover why acids and bases have a different ph level along with other important properties by looking at each substance. Properties of acids vs bases. common examples include vinegar (acetic acid, ch₃cooh), citrus fruits (citric acid, c₆h₈o₇), and stomach acid (hydrochloric acid, hcl). according to. Examples Of Acids Vs Bases.
From sciencenotes.org
Facts About Acids and Bases Examples Of Acids Vs Bases according to the lewis definition, acids are molecules or ions capable of coordinating with unshared electron pairs,. common examples include vinegar (acetic acid, ch₃cooh), citrus fruits (citric acid, c₆h₈o₇), and stomach acid (hydrochloric acid, hcl). discover why acids and bases have a different ph level along with other important properties by looking at each substance. the. Examples Of Acids Vs Bases.
From www.learnatnoon.com
What are the differences between Acids and Bases? Noon Examples Of Acids Vs Bases When equal moles of an acid and a base are combined, the acid is neutralized by the base. throughout history, chemists have created different definitions of acids and bases. the earliest definition of acids and bases is arrhenius's definition which states that: according to the lewis definition, acids are molecules or ions capable of coordinating with unshared. Examples Of Acids Vs Bases.
From sciencewithmrsb.weebly.com
Acids and Bases Science with Mrs Beggs Examples Of Acids Vs Bases according to the lewis definition, acids are molecules or ions capable of coordinating with unshared electron pairs,. Bases have a slippery feel on fingers and taste bitter. discover why acids and bases have a different ph level along with other important properties by looking at each substance. the earliest definition of acids and bases is arrhenius's definition. Examples Of Acids Vs Bases.
From shiken.ai
Weak Acids and Bases Examples Of Acids Vs Bases acids react with bases to produce a salt compound and water. discover why acids and bases have a different ph level along with other important properties by looking at each substance. the earliest definition of acids and bases is arrhenius's definition which states that: common examples include vinegar (acetic acid, ch₃cooh), citrus fruits (citric acid, c₆h₈o₇),. Examples Of Acids Vs Bases.
From sciencenotes.org
Lewis Acid and Base Theory Examples Of Acids Vs Bases Properties of acids vs bases. discover why acids and bases have a different ph level along with other important properties by looking at each substance. acids react with bases to produce a salt compound and water. throughout history, chemists have created different definitions of acids and bases. When equal moles of an acid and a base are. Examples Of Acids Vs Bases.
From www.homeworkjoy.com
Main Differences Between Acid and Base Examples Of Acids Vs Bases When equal moles of an acid and a base are combined, the acid is neutralized by the base. discover why acids and bases have a different ph level along with other important properties by looking at each substance. common examples include vinegar (acetic acid, ch₃cooh), citrus fruits (citric acid, c₆h₈o₇), and stomach acid (hydrochloric acid, hcl). acids. Examples Of Acids Vs Bases.
From www.teachoo.com
Difference between Acid and Base (in Table form) Teachoo Examples Of Acids Vs Bases according to the lewis definition, acids are molecules or ions capable of coordinating with unshared electron pairs,. common examples include vinegar (acetic acid, ch₃cooh), citrus fruits (citric acid, c₆h₈o₇), and stomach acid (hydrochloric acid, hcl). When equal moles of an acid and a base are combined, the acid is neutralized by the base. acids react with bases. Examples Of Acids Vs Bases.
From www.pinterest.ca
The following Venn diagram shows the similarities and differences between acids and bases. Ideal Examples Of Acids Vs Bases throughout history, chemists have created different definitions of acids and bases. according to the lewis definition, acids are molecules or ions capable of coordinating with unshared electron pairs,. An acid is a substance that forms hydrogen ions h + when. Properties of acids vs bases. acids react with bases to produce a salt compound and water. . Examples Of Acids Vs Bases.
From courses.lumenlearning.com
Relative Strengths of Acids and Bases Chemistry Examples Of Acids Vs Bases the earliest definition of acids and bases is arrhenius's definition which states that: common examples include vinegar (acetic acid, ch₃cooh), citrus fruits (citric acid, c₆h₈o₇), and stomach acid (hydrochloric acid, hcl). acids react with bases to produce a salt compound and water. discover why acids and bases have a different ph level along with other important. Examples Of Acids Vs Bases.
From www.chemicals.co.uk
A Level Chemistry Revision Physical Chemistry Acids And Bases Examples Of Acids Vs Bases the earliest definition of acids and bases is arrhenius's definition which states that: common examples include vinegar (acetic acid, ch₃cooh), citrus fruits (citric acid, c₆h₈o₇), and stomach acid (hydrochloric acid, hcl). An acid is a substance that forms hydrogen ions h + when. throughout history, chemists have created different definitions of acids and bases. Bases have a. Examples Of Acids Vs Bases.
From pediaa.com
Difference Between Acid and Base Examples Of Acids Vs Bases Bases have a slippery feel on fingers and taste bitter. Properties of acids vs bases. the earliest definition of acids and bases is arrhenius's definition which states that: common examples include vinegar (acetic acid, ch₃cooh), citrus fruits (citric acid, c₆h₈o₇), and stomach acid (hydrochloric acid, hcl). An acid is a substance that forms hydrogen ions h + when.. Examples Of Acids Vs Bases.
From blog.praxilabs.com
Learn All About The Strong Acids and Bases PraxiLabs Examples Of Acids Vs Bases acids react with bases to produce a salt compound and water. discover why acids and bases have a different ph level along with other important properties by looking at each substance. Properties of acids vs bases. When equal moles of an acid and a base are combined, the acid is neutralized by the base. common examples include. Examples Of Acids Vs Bases.
From www.dreamstime.com
Acids, Neutral and Bases Substances Scale with Examples Outline Diagram Stock Vector Examples Of Acids Vs Bases An acid is a substance that forms hydrogen ions h + when. When equal moles of an acid and a base are combined, the acid is neutralized by the base. Bases have a slippery feel on fingers and taste bitter. discover why acids and bases have a different ph level along with other important properties by looking at each. Examples Of Acids Vs Bases.
From www.haikudeck.com
Acid vs Bases by Isaac And Greg Examples Of Acids Vs Bases Properties of acids vs bases. When equal moles of an acid and a base are combined, the acid is neutralized by the base. discover why acids and bases have a different ph level along with other important properties by looking at each substance. according to the lewis definition, acids are molecules or ions capable of coordinating with unshared. Examples Of Acids Vs Bases.
From www.chemistrysteps.com
Lewis Acids and Bases Chemistry Steps Examples Of Acids Vs Bases An acid is a substance that forms hydrogen ions h + when. Properties of acids vs bases. according to the lewis definition, acids are molecules or ions capable of coordinating with unshared electron pairs,. common examples include vinegar (acetic acid, ch₃cooh), citrus fruits (citric acid, c₆h₈o₇), and stomach acid (hydrochloric acid, hcl). discover why acids and bases. Examples Of Acids Vs Bases.
From stock.adobe.com
Vetor de Acids Bases and Salts infographic diagram showing solution with comparison table and Examples Of Acids Vs Bases discover why acids and bases have a different ph level along with other important properties by looking at each substance. When equal moles of an acid and a base are combined, the acid is neutralized by the base. Properties of acids vs bases. throughout history, chemists have created different definitions of acids and bases. An acid is a. Examples Of Acids Vs Bases.
From ar.inspiredpencil.com
Acids And Bases List Examples Of Acids Vs Bases An acid is a substance that forms hydrogen ions h + when. according to the lewis definition, acids are molecules or ions capable of coordinating with unshared electron pairs,. common examples include vinegar (acetic acid, ch₃cooh), citrus fruits (citric acid, c₆h₈o₇), and stomach acid (hydrochloric acid, hcl). Properties of acids vs bases. Bases have a slippery feel on. Examples Of Acids Vs Bases.
From www.expii.com
Lewis Acid and Bases — Definition & Examples Expii Examples Of Acids Vs Bases When equal moles of an acid and a base are combined, the acid is neutralized by the base. throughout history, chemists have created different definitions of acids and bases. according to the lewis definition, acids are molecules or ions capable of coordinating with unshared electron pairs,. Bases have a slippery feel on fingers and taste bitter. discover. Examples Of Acids Vs Bases.
From leah4sci.com
Definitions of Arrhenius, BronstedLowry, and Lewis Acids and Bases in Organic Chemistry Examples Of Acids Vs Bases common examples include vinegar (acetic acid, ch₃cooh), citrus fruits (citric acid, c₆h₈o₇), and stomach acid (hydrochloric acid, hcl). Properties of acids vs bases. When equal moles of an acid and a base are combined, the acid is neutralized by the base. Bases have a slippery feel on fingers and taste bitter. the earliest definition of acids and bases. Examples Of Acids Vs Bases.
From praxilabs.com
Learn All About The Strong Acids and Bases PraxiLabs Examples Of Acids Vs Bases common examples include vinegar (acetic acid, ch₃cooh), citrus fruits (citric acid, c₆h₈o₇), and stomach acid (hydrochloric acid, hcl). acids react with bases to produce a salt compound and water. When equal moles of an acid and a base are combined, the acid is neutralized by the base. throughout history, chemists have created different definitions of acids and. Examples Of Acids Vs Bases.
From scienceandsf.com
Acids, Bases and pH. Scienceandsf A Blog Published by Robert A. Lawler Examples Of Acids Vs Bases Bases have a slippery feel on fingers and taste bitter. according to the lewis definition, acids are molecules or ions capable of coordinating with unshared electron pairs,. An acid is a substance that forms hydrogen ions h + when. common examples include vinegar (acetic acid, ch₃cooh), citrus fruits (citric acid, c₆h₈o₇), and stomach acid (hydrochloric acid, hcl). When. Examples Of Acids Vs Bases.
From mungfali.com
Acid Base Concept Map Examples Of Acids Vs Bases according to the lewis definition, acids are molecules or ions capable of coordinating with unshared electron pairs,. An acid is a substance that forms hydrogen ions h + when. When equal moles of an acid and a base are combined, the acid is neutralized by the base. throughout history, chemists have created different definitions of acids and bases.. Examples Of Acids Vs Bases.
From dashamlav.com
Acid vs Base Table of Differences between Acid and Base Examples Of Acids Vs Bases discover why acids and bases have a different ph level along with other important properties by looking at each substance. acids react with bases to produce a salt compound and water. the earliest definition of acids and bases is arrhenius's definition which states that: An acid is a substance that forms hydrogen ions h + when. When. Examples Of Acids Vs Bases.
From morioh.com
Acids and Bases Basic Introduction Chemistry Examples Of Acids Vs Bases common examples include vinegar (acetic acid, ch₃cooh), citrus fruits (citric acid, c₆h₈o₇), and stomach acid (hydrochloric acid, hcl). according to the lewis definition, acids are molecules or ions capable of coordinating with unshared electron pairs,. Bases have a slippery feel on fingers and taste bitter. discover why acids and bases have a different ph level along with. Examples Of Acids Vs Bases.
From byjus.com
Difference between Acid and Base Differences btw Acid & Base in Tabular Form Examples Of Acids Vs Bases Bases have a slippery feel on fingers and taste bitter. the earliest definition of acids and bases is arrhenius's definition which states that: according to the lewis definition, acids are molecules or ions capable of coordinating with unshared electron pairs,. An acid is a substance that forms hydrogen ions h + when. Properties of acids vs bases. . Examples Of Acids Vs Bases.
From www.teachoo.com
Strength of Acids and Bases (How to find it?) Chemistry Teachoo Examples Of Acids Vs Bases common examples include vinegar (acetic acid, ch₃cooh), citrus fruits (citric acid, c₆h₈o₇), and stomach acid (hydrochloric acid, hcl). Properties of acids vs bases. acids react with bases to produce a salt compound and water. When equal moles of an acid and a base are combined, the acid is neutralized by the base. discover why acids and bases. Examples Of Acids Vs Bases.
From www.pinterest.com
Lewis Acids and Bases Lewis acids and bases, Organic reactions, Chemistry Examples Of Acids Vs Bases discover why acids and bases have a different ph level along with other important properties by looking at each substance. acids react with bases to produce a salt compound and water. common examples include vinegar (acetic acid, ch₃cooh), citrus fruits (citric acid, c₆h₈o₇), and stomach acid (hydrochloric acid, hcl). An acid is a substance that forms hydrogen. Examples Of Acids Vs Bases.
From www.teachoo.com
What do all Acids and Bases have in common? (with 5+ Points) Teachoo Examples Of Acids Vs Bases An acid is a substance that forms hydrogen ions h + when. acids react with bases to produce a salt compound and water. throughout history, chemists have created different definitions of acids and bases. common examples include vinegar (acetic acid, ch₃cooh), citrus fruits (citric acid, c₆h₈o₇), and stomach acid (hydrochloric acid, hcl). Properties of acids vs bases.. Examples Of Acids Vs Bases.
From www.worksheetsplanet.com
Acid and Bases Differences Examples Of Acids Vs Bases When equal moles of an acid and a base are combined, the acid is neutralized by the base. Properties of acids vs bases. according to the lewis definition, acids are molecules or ions capable of coordinating with unshared electron pairs,. acids react with bases to produce a salt compound and water. discover why acids and bases have. Examples Of Acids Vs Bases.