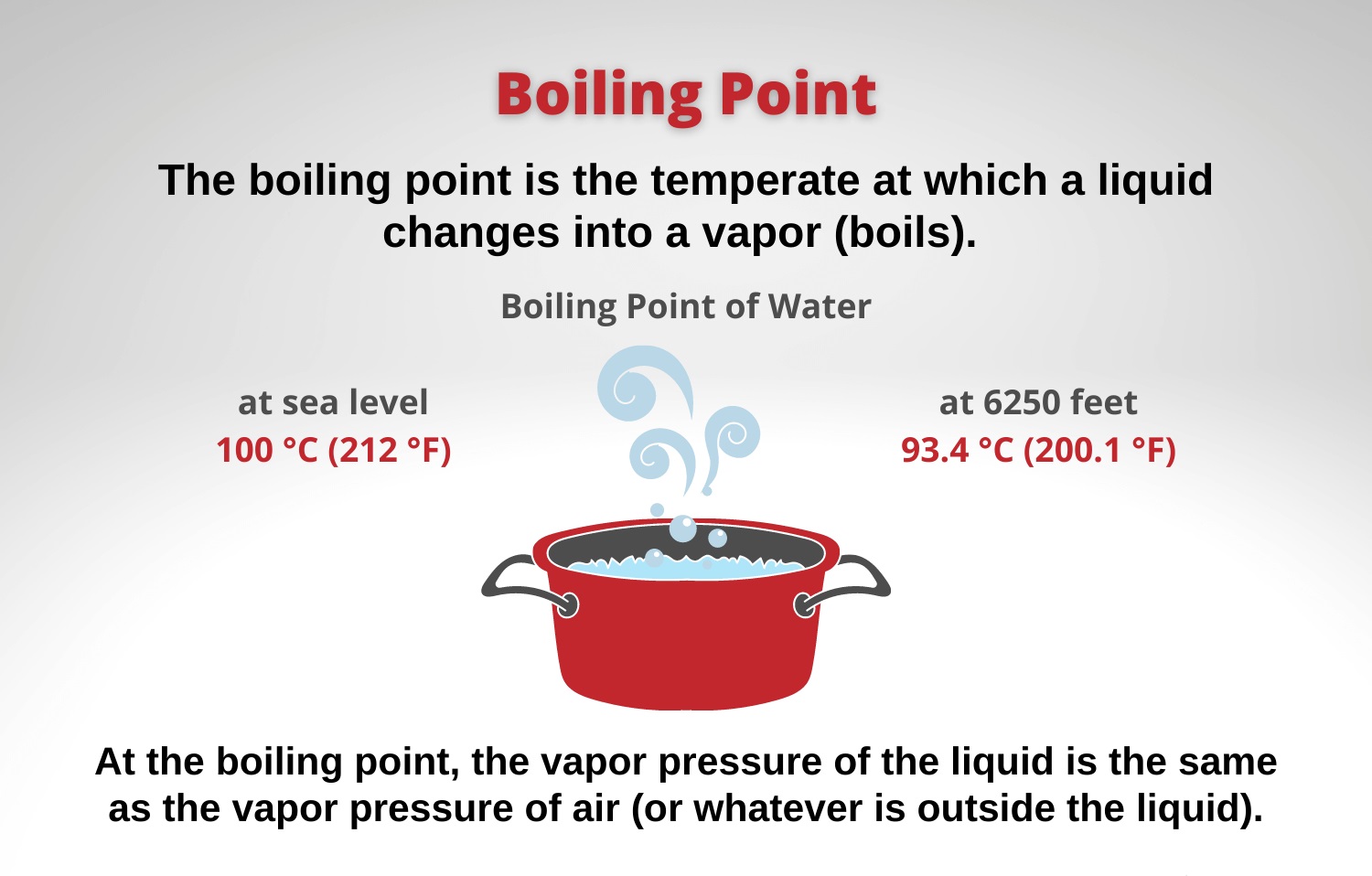
Why Is Chlorine Boiling Point Low . Sodium chloride (nacl) is a. Chlorine (cl2) is a gas at room temperature because it has weak intermolecular forces and a low boiling point. Sodium chloride is a solid because it has. Chlorine has a boiling point of $238~\mathrm{k}$ while hydrogen chloride has a boiling point of $188~\mathrm{k}$. As a result, chlorine is smaller and has a smaller atomic radius. Chlorine is a gas at room temperature because it has weak intermolecular forces and a low boiling point. The relative strength of the four intermolecular forces. Learn about the physical and chemical properties of chlorine, a greenish yellow gas with a penetrating and irritating odor. The boiling point of a liquid depends on the intermolecular forces present between the atoms or molecules in the liquid since you must disrupt those forces to change from a liquid. Chlorine, as chlorine has fewer electrons shells than bromine.
from facts.net
Chlorine (cl2) is a gas at room temperature because it has weak intermolecular forces and a low boiling point. Sodium chloride is a solid because it has. As a result, chlorine is smaller and has a smaller atomic radius. Learn about the physical and chemical properties of chlorine, a greenish yellow gas with a penetrating and irritating odor. The relative strength of the four intermolecular forces. Sodium chloride (nacl) is a. The boiling point of a liquid depends on the intermolecular forces present between the atoms or molecules in the liquid since you must disrupt those forces to change from a liquid. Chlorine, as chlorine has fewer electrons shells than bromine. Chlorine has a boiling point of $238~\mathrm{k}$ while hydrogen chloride has a boiling point of $188~\mathrm{k}$. Chlorine is a gas at room temperature because it has weak intermolecular forces and a low boiling point.
20 Fascinating Facts About Boiling Point
Why Is Chlorine Boiling Point Low Learn about the physical and chemical properties of chlorine, a greenish yellow gas with a penetrating and irritating odor. Learn about the physical and chemical properties of chlorine, a greenish yellow gas with a penetrating and irritating odor. Chlorine (cl2) is a gas at room temperature because it has weak intermolecular forces and a low boiling point. Sodium chloride (nacl) is a. The boiling point of a liquid depends on the intermolecular forces present between the atoms or molecules in the liquid since you must disrupt those forces to change from a liquid. The relative strength of the four intermolecular forces. As a result, chlorine is smaller and has a smaller atomic radius. Sodium chloride is a solid because it has. Chlorine, as chlorine has fewer electrons shells than bromine. Chlorine is a gas at room temperature because it has weak intermolecular forces and a low boiling point. Chlorine has a boiling point of $238~\mathrm{k}$ while hydrogen chloride has a boiling point of $188~\mathrm{k}$.
From www.techquintal.com
10 Advantages and Disadvantages of Chlorination of Water to Know Tech Why Is Chlorine Boiling Point Low Sodium chloride is a solid because it has. Chlorine, as chlorine has fewer electrons shells than bromine. The boiling point of a liquid depends on the intermolecular forces present between the atoms or molecules in the liquid since you must disrupt those forces to change from a liquid. Chlorine is a gas at room temperature because it has weak intermolecular. Why Is Chlorine Boiling Point Low.
From peacecommission.kdsg.gov.ng
Oxygen Boiling Point Why Is Chlorine Boiling Point Low The relative strength of the four intermolecular forces. As a result, chlorine is smaller and has a smaller atomic radius. Sodium chloride (nacl) is a. Sodium chloride is a solid because it has. Chlorine has a boiling point of $238~\mathrm{k}$ while hydrogen chloride has a boiling point of $188~\mathrm{k}$. Chlorine is a gas at room temperature because it has weak. Why Is Chlorine Boiling Point Low.
From jsmithmoore.com
Boiling point of ethanol celsius Why Is Chlorine Boiling Point Low The boiling point of a liquid depends on the intermolecular forces present between the atoms or molecules in the liquid since you must disrupt those forces to change from a liquid. Learn about the physical and chemical properties of chlorine, a greenish yellow gas with a penetrating and irritating odor. Sodium chloride is a solid because it has. Chlorine (cl2). Why Is Chlorine Boiling Point Low.
From fyouzbxlm.blob.core.windows.net
Why Does Chlorine Boiling Point at Hector Duerr blog Why Is Chlorine Boiling Point Low The boiling point of a liquid depends on the intermolecular forces present between the atoms or molecules in the liquid since you must disrupt those forces to change from a liquid. Chlorine, as chlorine has fewer electrons shells than bromine. As a result, chlorine is smaller and has a smaller atomic radius. Chlorine (cl2) is a gas at room temperature. Why Is Chlorine Boiling Point Low.
From www.chemicals.co.uk
A Level Chemistry Revision Organic Chemistry Alcohols Why Is Chlorine Boiling Point Low Learn about the physical and chemical properties of chlorine, a greenish yellow gas with a penetrating and irritating odor. Chlorine (cl2) is a gas at room temperature because it has weak intermolecular forces and a low boiling point. Sodium chloride (nacl) is a. Chlorine has a boiling point of $238~\mathrm{k}$ while hydrogen chloride has a boiling point of $188~\mathrm{k}$. The. Why Is Chlorine Boiling Point Low.
From slidetodoc.com
CHLORINATION Why We Use Chlorine Chlorine is the Why Is Chlorine Boiling Point Low Chlorine has a boiling point of $238~\mathrm{k}$ while hydrogen chloride has a boiling point of $188~\mathrm{k}$. The boiling point of a liquid depends on the intermolecular forces present between the atoms or molecules in the liquid since you must disrupt those forces to change from a liquid. Sodium chloride is a solid because it has. Learn about the physical and. Why Is Chlorine Boiling Point Low.
From hxewsjoxk.blob.core.windows.net
Boiling Point Chlorine Methane at Mary Suttles blog Why Is Chlorine Boiling Point Low The boiling point of a liquid depends on the intermolecular forces present between the atoms or molecules in the liquid since you must disrupt those forces to change from a liquid. Chlorine has a boiling point of $238~\mathrm{k}$ while hydrogen chloride has a boiling point of $188~\mathrm{k}$. Chlorine, as chlorine has fewer electrons shells than bromine. Chlorine is a gas. Why Is Chlorine Boiling Point Low.
From www.worksheetsplanet.com
What is Boiling Point Why Is Chlorine Boiling Point Low The relative strength of the four intermolecular forces. Chlorine (cl2) is a gas at room temperature because it has weak intermolecular forces and a low boiling point. Chlorine is a gas at room temperature because it has weak intermolecular forces and a low boiling point. The boiling point of a liquid depends on the intermolecular forces present between the atoms. Why Is Chlorine Boiling Point Low.
From brainly.in
why does temperature remain constant at the melting point or boiling Why Is Chlorine Boiling Point Low As a result, chlorine is smaller and has a smaller atomic radius. The boiling point of a liquid depends on the intermolecular forces present between the atoms or molecules in the liquid since you must disrupt those forces to change from a liquid. Sodium chloride (nacl) is a. Chlorine is a gas at room temperature because it has weak intermolecular. Why Is Chlorine Boiling Point Low.
From www.slideshare.net
Chlorine Hazards 2009 Why Is Chlorine Boiling Point Low Sodium chloride is a solid because it has. Chlorine (cl2) is a gas at room temperature because it has weak intermolecular forces and a low boiling point. Chlorine is a gas at room temperature because it has weak intermolecular forces and a low boiling point. Chlorine, as chlorine has fewer electrons shells than bromine. The relative strength of the four. Why Is Chlorine Boiling Point Low.
From www.slideserve.com
PPT MELTING AND BOILING PowerPoint Presentation, free download ID Why Is Chlorine Boiling Point Low Learn about the physical and chemical properties of chlorine, a greenish yellow gas with a penetrating and irritating odor. The relative strength of the four intermolecular forces. The boiling point of a liquid depends on the intermolecular forces present between the atoms or molecules in the liquid since you must disrupt those forces to change from a liquid. As a. Why Is Chlorine Boiling Point Low.
From www.haikudeck.com
The Element Chlorine by Mary Won Why Is Chlorine Boiling Point Low Chlorine (cl2) is a gas at room temperature because it has weak intermolecular forces and a low boiling point. Chlorine is a gas at room temperature because it has weak intermolecular forces and a low boiling point. Chlorine has a boiling point of $238~\mathrm{k}$ while hydrogen chloride has a boiling point of $188~\mathrm{k}$. Learn about the physical and chemical properties. Why Is Chlorine Boiling Point Low.
From purewaterblog.com
Does Boiling Water Remove Chlorine from Drinking Water? Water Treatment Why Is Chlorine Boiling Point Low Sodium chloride (nacl) is a. As a result, chlorine is smaller and has a smaller atomic radius. Sodium chloride is a solid because it has. Chlorine, as chlorine has fewer electrons shells than bromine. Chlorine has a boiling point of $238~\mathrm{k}$ while hydrogen chloride has a boiling point of $188~\mathrm{k}$. The relative strength of the four intermolecular forces. Chlorine is. Why Is Chlorine Boiling Point Low.
From waterfilterguru.com
Does Boiling Water Remove Chlorine? (Find Out Here) Why Is Chlorine Boiling Point Low Chlorine, as chlorine has fewer electrons shells than bromine. As a result, chlorine is smaller and has a smaller atomic radius. Sodium chloride (nacl) is a. Sodium chloride is a solid because it has. Learn about the physical and chemical properties of chlorine, a greenish yellow gas with a penetrating and irritating odor. The boiling point of a liquid depends. Why Is Chlorine Boiling Point Low.
From www.researchgate.net
The saturation curve of pressure against temperature for chlorine Why Is Chlorine Boiling Point Low Chlorine is a gas at room temperature because it has weak intermolecular forces and a low boiling point. The relative strength of the four intermolecular forces. As a result, chlorine is smaller and has a smaller atomic radius. Sodium chloride is a solid because it has. The boiling point of a liquid depends on the intermolecular forces present between the. Why Is Chlorine Boiling Point Low.
From fyouzbxlm.blob.core.windows.net
Why Does Chlorine Boiling Point at Hector Duerr blog Why Is Chlorine Boiling Point Low Chlorine (cl2) is a gas at room temperature because it has weak intermolecular forces and a low boiling point. As a result, chlorine is smaller and has a smaller atomic radius. The boiling point of a liquid depends on the intermolecular forces present between the atoms or molecules in the liquid since you must disrupt those forces to change from. Why Is Chlorine Boiling Point Low.
From dkhfxaogeco.blob.core.windows.net
Chlorine Higher Boiling Point Than Iodine at Benjamin Lakin blog Why Is Chlorine Boiling Point Low Sodium chloride is a solid because it has. Chlorine (cl2) is a gas at room temperature because it has weak intermolecular forces and a low boiling point. Learn about the physical and chemical properties of chlorine, a greenish yellow gas with a penetrating and irritating odor. The relative strength of the four intermolecular forces. Chlorine, as chlorine has fewer electrons. Why Is Chlorine Boiling Point Low.
From gabriela-classwork.blogspot.com
Science Class septiembre 2010 Why Is Chlorine Boiling Point Low The boiling point of a liquid depends on the intermolecular forces present between the atoms or molecules in the liquid since you must disrupt those forces to change from a liquid. Chlorine, as chlorine has fewer electrons shells than bromine. Chlorine is a gas at room temperature because it has weak intermolecular forces and a low boiling point. Sodium chloride. Why Is Chlorine Boiling Point Low.
From pediaa.com
Difference Between Vapor Pressure and Boiling Point Definition Why Is Chlorine Boiling Point Low Chlorine is a gas at room temperature because it has weak intermolecular forces and a low boiling point. Chlorine, as chlorine has fewer electrons shells than bromine. Sodium chloride is a solid because it has. Sodium chloride (nacl) is a. Chlorine has a boiling point of $238~\mathrm{k}$ while hydrogen chloride has a boiling point of $188~\mathrm{k}$. Learn about the physical. Why Is Chlorine Boiling Point Low.
From diagramfricanofc.z21.web.core.windows.net
Initial Boiling Point And Final Boiling Point Why Is Chlorine Boiling Point Low Learn about the physical and chemical properties of chlorine, a greenish yellow gas with a penetrating and irritating odor. Chlorine has a boiling point of $238~\mathrm{k}$ while hydrogen chloride has a boiling point of $188~\mathrm{k}$. Sodium chloride (nacl) is a. Chlorine, as chlorine has fewer electrons shells than bromine. As a result, chlorine is smaller and has a smaller atomic. Why Is Chlorine Boiling Point Low.
From ivypanda.com
Massive Leak of Liquified Chlorine Gas 2169 Words Presentation Example Why Is Chlorine Boiling Point Low Sodium chloride is a solid because it has. The boiling point of a liquid depends on the intermolecular forces present between the atoms or molecules in the liquid since you must disrupt those forces to change from a liquid. The relative strength of the four intermolecular forces. As a result, chlorine is smaller and has a smaller atomic radius. Chlorine. Why Is Chlorine Boiling Point Low.
From www.nagwa.com
Question Video Determining Why Covalent Compounds Have Low Melting and Why Is Chlorine Boiling Point Low Sodium chloride (nacl) is a. Chlorine is a gas at room temperature because it has weak intermolecular forces and a low boiling point. Chlorine (cl2) is a gas at room temperature because it has weak intermolecular forces and a low boiling point. Sodium chloride is a solid because it has. The relative strength of the four intermolecular forces. The boiling. Why Is Chlorine Boiling Point Low.
From www.youtube.com
Q. Why boiling point of water is greater than hydrogen sulphide?( Class Why Is Chlorine Boiling Point Low Sodium chloride (nacl) is a. Learn about the physical and chemical properties of chlorine, a greenish yellow gas with a penetrating and irritating odor. Sodium chloride is a solid because it has. The boiling point of a liquid depends on the intermolecular forces present between the atoms or molecules in the liquid since you must disrupt those forces to change. Why Is Chlorine Boiling Point Low.
From www.youtube.com
Boiling Point of NH3 and H2O (Explanation of Difference) YouTube Why Is Chlorine Boiling Point Low Sodium chloride (nacl) is a. The boiling point of a liquid depends on the intermolecular forces present between the atoms or molecules in the liquid since you must disrupt those forces to change from a liquid. Chlorine has a boiling point of $238~\mathrm{k}$ while hydrogen chloride has a boiling point of $188~\mathrm{k}$. Chlorine (cl2) is a gas at room temperature. Why Is Chlorine Boiling Point Low.
From material-properties.org
Chlorine Thermal Properties Melting Point Thermal Conductivity Why Is Chlorine Boiling Point Low The boiling point of a liquid depends on the intermolecular forces present between the atoms or molecules in the liquid since you must disrupt those forces to change from a liquid. The relative strength of the four intermolecular forces. Chlorine (cl2) is a gas at room temperature because it has weak intermolecular forces and a low boiling point. Chlorine, as. Why Is Chlorine Boiling Point Low.
From www.youtube.com
Why aldehyde ketones have lower boiling point than alcohol, carboxylic Why Is Chlorine Boiling Point Low As a result, chlorine is smaller and has a smaller atomic radius. Chlorine (cl2) is a gas at room temperature because it has weak intermolecular forces and a low boiling point. Learn about the physical and chemical properties of chlorine, a greenish yellow gas with a penetrating and irritating odor. Chlorine is a gas at room temperature because it has. Why Is Chlorine Boiling Point Low.
From chemizi.blogspot.com
Why boiling point of alcohol is higher than ether and alkane Why Is Chlorine Boiling Point Low The relative strength of the four intermolecular forces. The boiling point of a liquid depends on the intermolecular forces present between the atoms or molecules in the liquid since you must disrupt those forces to change from a liquid. Chlorine, as chlorine has fewer electrons shells than bromine. Chlorine (cl2) is a gas at room temperature because it has weak. Why Is Chlorine Boiling Point Low.
From sciencenotes.org
Boiling Point of Water What Temperature Does Water Boil? Why Is Chlorine Boiling Point Low As a result, chlorine is smaller and has a smaller atomic radius. Sodium chloride (nacl) is a. Chlorine has a boiling point of $238~\mathrm{k}$ while hydrogen chloride has a boiling point of $188~\mathrm{k}$. The boiling point of a liquid depends on the intermolecular forces present between the atoms or molecules in the liquid since you must disrupt those forces to. Why Is Chlorine Boiling Point Low.
From www.learnatnoon.com
The boiling point of water and alcohol explained Noon Academy Why Is Chlorine Boiling Point Low The boiling point of a liquid depends on the intermolecular forces present between the atoms or molecules in the liquid since you must disrupt those forces to change from a liquid. Sodium chloride is a solid because it has. The relative strength of the four intermolecular forces. Chlorine is a gas at room temperature because it has weak intermolecular forces. Why Is Chlorine Boiling Point Low.
From www.nuclear-power.com
Chlorine Melting Point Boiling Point Why Is Chlorine Boiling Point Low As a result, chlorine is smaller and has a smaller atomic radius. Chlorine has a boiling point of $238~\mathrm{k}$ while hydrogen chloride has a boiling point of $188~\mathrm{k}$. The relative strength of the four intermolecular forces. The boiling point of a liquid depends on the intermolecular forces present between the atoms or molecules in the liquid since you must disrupt. Why Is Chlorine Boiling Point Low.
From exobnyect.blob.core.windows.net
Why Does Steam Distillation Lower Boiling Point at Harold Sheehan blog Why Is Chlorine Boiling Point Low Sodium chloride (nacl) is a. Sodium chloride is a solid because it has. Chlorine has a boiling point of $238~\mathrm{k}$ while hydrogen chloride has a boiling point of $188~\mathrm{k}$. As a result, chlorine is smaller and has a smaller atomic radius. The relative strength of the four intermolecular forces. Learn about the physical and chemical properties of chlorine, a greenish. Why Is Chlorine Boiling Point Low.
From facts.net
20 Fascinating Facts About Boiling Point Why Is Chlorine Boiling Point Low The boiling point of a liquid depends on the intermolecular forces present between the atoms or molecules in the liquid since you must disrupt those forces to change from a liquid. Chlorine is a gas at room temperature because it has weak intermolecular forces and a low boiling point. Learn about the physical and chemical properties of chlorine, a greenish. Why Is Chlorine Boiling Point Low.
From waterfilterspot.com
Does Boiling Water Remove Chlorine From Water? (Quick Answer) Why Is Chlorine Boiling Point Low Chlorine, as chlorine has fewer electrons shells than bromine. Sodium chloride is a solid because it has. Chlorine has a boiling point of $238~\mathrm{k}$ while hydrogen chloride has a boiling point of $188~\mathrm{k}$. Learn about the physical and chemical properties of chlorine, a greenish yellow gas with a penetrating and irritating odor. The boiling point of a liquid depends on. Why Is Chlorine Boiling Point Low.
From dkhfxaogeco.blob.core.windows.net
Chlorine Higher Boiling Point Than Iodine at Benjamin Lakin blog Why Is Chlorine Boiling Point Low Chlorine, as chlorine has fewer electrons shells than bromine. Chlorine (cl2) is a gas at room temperature because it has weak intermolecular forces and a low boiling point. The boiling point of a liquid depends on the intermolecular forces present between the atoms or molecules in the liquid since you must disrupt those forces to change from a liquid. The. Why Is Chlorine Boiling Point Low.
From fyouzbxlm.blob.core.windows.net
Why Does Chlorine Boiling Point at Hector Duerr blog Why Is Chlorine Boiling Point Low Chlorine, as chlorine has fewer electrons shells than bromine. The relative strength of the four intermolecular forces. Chlorine is a gas at room temperature because it has weak intermolecular forces and a low boiling point. The boiling point of a liquid depends on the intermolecular forces present between the atoms or molecules in the liquid since you must disrupt those. Why Is Chlorine Boiling Point Low.