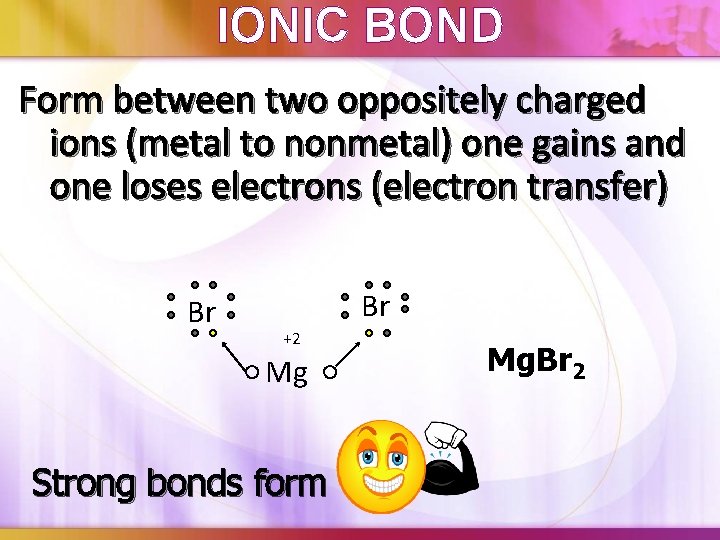
Ionic Bond Metal To Nonmetal . Electronegativity is a property of an atom, measuring how strongly it attracts or. A metal bonding to a nonmetal forms an ionic bond, while two nonmetals form a covalent bond. There are four kinds of bonding types to be aware of. Otherwise, consult a chart of electronegativity values. A metal (which forms the cations) and a nonmetal (which forms the anions). How do ionic bonds form. By definition, a metal is relatively stable if it loses electrons to form a complete valence shell and becomes positively charged. Ionic bonding forms between two atoms when an electron is transferred from one. Binary ionic compounds are composed of just two elements:
from slidetodoc.com
Binary ionic compounds are composed of just two elements: By definition, a metal is relatively stable if it loses electrons to form a complete valence shell and becomes positively charged. Otherwise, consult a chart of electronegativity values. Electronegativity is a property of an atom, measuring how strongly it attracts or. A metal (which forms the cations) and a nonmetal (which forms the anions). How do ionic bonds form. A metal bonding to a nonmetal forms an ionic bond, while two nonmetals form a covalent bond. Ionic bonding forms between two atoms when an electron is transferred from one. There are four kinds of bonding types to be aware of.
FLASHBACK How many electrons do all atoms want
Ionic Bond Metal To Nonmetal Binary ionic compounds are composed of just two elements: By definition, a metal is relatively stable if it loses electrons to form a complete valence shell and becomes positively charged. Ionic bonding forms between two atoms when an electron is transferred from one. Binary ionic compounds are composed of just two elements: There are four kinds of bonding types to be aware of. A metal bonding to a nonmetal forms an ionic bond, while two nonmetals form a covalent bond. Electronegativity is a property of an atom, measuring how strongly it attracts or. Otherwise, consult a chart of electronegativity values. A metal (which forms the cations) and a nonmetal (which forms the anions). How do ionic bonds form.
From slideplayer.com
13.1 Electrons and Chemical Bonds ppt download Ionic Bond Metal To Nonmetal How do ionic bonds form. Ionic bonding forms between two atoms when an electron is transferred from one. There are four kinds of bonding types to be aware of. Otherwise, consult a chart of electronegativity values. A metal bonding to a nonmetal forms an ionic bond, while two nonmetals form a covalent bond. Electronegativity is a property of an atom,. Ionic Bond Metal To Nonmetal.
From slideplayer.com
ATOM defined by number of p+ behavior described by n, e ppt download Ionic Bond Metal To Nonmetal Electronegativity is a property of an atom, measuring how strongly it attracts or. Ionic bonding forms between two atoms when an electron is transferred from one. A metal (which forms the cations) and a nonmetal (which forms the anions). Otherwise, consult a chart of electronegativity values. Binary ionic compounds are composed of just two elements: By definition, a metal is. Ionic Bond Metal To Nonmetal.
From slideplayer.com
Bonding Ionic bond (formula units) Between metal and a nonmetal ppt Ionic Bond Metal To Nonmetal A metal (which forms the cations) and a nonmetal (which forms the anions). Ionic bonding forms between two atoms when an electron is transferred from one. There are four kinds of bonding types to be aware of. Binary ionic compounds are composed of just two elements: A metal bonding to a nonmetal forms an ionic bond, while two nonmetals form. Ionic Bond Metal To Nonmetal.
From slideplayer.com
CHEMICAL BONDING Cocaine ppt download Ionic Bond Metal To Nonmetal A metal (which forms the cations) and a nonmetal (which forms the anions). Ionic bonding forms between two atoms when an electron is transferred from one. By definition, a metal is relatively stable if it loses electrons to form a complete valence shell and becomes positively charged. Otherwise, consult a chart of electronegativity values. A metal bonding to a nonmetal. Ionic Bond Metal To Nonmetal.
From slideplayer.com
The Chemical Nature of Matter 7.P.2A.4 Notes. ppt download Ionic Bond Metal To Nonmetal Binary ionic compounds are composed of just two elements: A metal bonding to a nonmetal forms an ionic bond, while two nonmetals form a covalent bond. How do ionic bonds form. By definition, a metal is relatively stable if it loses electrons to form a complete valence shell and becomes positively charged. There are four kinds of bonding types to. Ionic Bond Metal To Nonmetal.
From slideplayer.com
IONIC BONDS What are Ionic Bonds? Writing Compounds. ppt download Ionic Bond Metal To Nonmetal A metal bonding to a nonmetal forms an ionic bond, while two nonmetals form a covalent bond. Otherwise, consult a chart of electronegativity values. How do ionic bonds form. Binary ionic compounds are composed of just two elements: A metal (which forms the cations) and a nonmetal (which forms the anions). Electronegativity is a property of an atom, measuring how. Ionic Bond Metal To Nonmetal.
From www.slideserve.com
PPT Ionic Compounds Formula to Name PowerPoint Presentation, free Ionic Bond Metal To Nonmetal By definition, a metal is relatively stable if it loses electrons to form a complete valence shell and becomes positively charged. Otherwise, consult a chart of electronegativity values. How do ionic bonds form. There are four kinds of bonding types to be aware of. Electronegativity is a property of an atom, measuring how strongly it attracts or. A metal bonding. Ionic Bond Metal To Nonmetal.
From jaylenyouthmejia.blogspot.com
Ionic Bonding Between Metals and Nonmetals Ionic Bond Metal To Nonmetal Otherwise, consult a chart of electronegativity values. By definition, a metal is relatively stable if it loses electrons to form a complete valence shell and becomes positively charged. A metal bonding to a nonmetal forms an ionic bond, while two nonmetals form a covalent bond. Electronegativity is a property of an atom, measuring how strongly it attracts or. Binary ionic. Ionic Bond Metal To Nonmetal.
From www.slideserve.com
PPT Ionic and Metallic Bonding PowerPoint Presentation, free download Ionic Bond Metal To Nonmetal Binary ionic compounds are composed of just two elements: There are four kinds of bonding types to be aware of. Ionic bonding forms between two atoms when an electron is transferred from one. Otherwise, consult a chart of electronegativity values. How do ionic bonds form. By definition, a metal is relatively stable if it loses electrons to form a complete. Ionic Bond Metal To Nonmetal.
From www.chemistrylearner.com
Ionic, Covalent, and Metallic Bonds Differences and Similarities Ionic Bond Metal To Nonmetal How do ionic bonds form. Ionic bonding forms between two atoms when an electron is transferred from one. A metal (which forms the cations) and a nonmetal (which forms the anions). There are four kinds of bonding types to be aware of. Binary ionic compounds are composed of just two elements: Otherwise, consult a chart of electronegativity values. Electronegativity is. Ionic Bond Metal To Nonmetal.
From slideplayer.com
What is an Ionic Bond?. Chapter 15 Ionic Bonding and Ionic Compounds Ionic Bond Metal To Nonmetal Binary ionic compounds are composed of just two elements: A metal (which forms the cations) and a nonmetal (which forms the anions). By definition, a metal is relatively stable if it loses electrons to form a complete valence shell and becomes positively charged. Otherwise, consult a chart of electronegativity values. Electronegativity is a property of an atom, measuring how strongly. Ionic Bond Metal To Nonmetal.
From www.slideserve.com
PPT Writing Chemical Formulas and Naming Chemical Compounds Ionic Bond Metal To Nonmetal A metal bonding to a nonmetal forms an ionic bond, while two nonmetals form a covalent bond. A metal (which forms the cations) and a nonmetal (which forms the anions). Ionic bonding forms between two atoms when an electron is transferred from one. Electronegativity is a property of an atom, measuring how strongly it attracts or. Binary ionic compounds are. Ionic Bond Metal To Nonmetal.
From www.sliderbase.com
Ionic Bonding Presentation Chemistry Ionic Bond Metal To Nonmetal There are four kinds of bonding types to be aware of. Ionic bonding forms between two atoms when an electron is transferred from one. How do ionic bonds form. Otherwise, consult a chart of electronegativity values. Electronegativity is a property of an atom, measuring how strongly it attracts or. A metal bonding to a nonmetal forms an ionic bond, while. Ionic Bond Metal To Nonmetal.
From slideplayer.com
II. Kinds of Chemical Bonds (p ) ppt download Ionic Bond Metal To Nonmetal Binary ionic compounds are composed of just two elements: A metal (which forms the cations) and a nonmetal (which forms the anions). Ionic bonding forms between two atoms when an electron is transferred from one. A metal bonding to a nonmetal forms an ionic bond, while two nonmetals form a covalent bond. There are four kinds of bonding types to. Ionic Bond Metal To Nonmetal.
From slideplayer.com
Chapter 9 Chemical Bonding I Lewis Theory ppt download Ionic Bond Metal To Nonmetal A metal (which forms the cations) and a nonmetal (which forms the anions). By definition, a metal is relatively stable if it loses electrons to form a complete valence shell and becomes positively charged. There are four kinds of bonding types to be aware of. Otherwise, consult a chart of electronegativity values. Electronegativity is a property of an atom, measuring. Ionic Bond Metal To Nonmetal.
From en.wikipedia.org
Ionic bonding Wikipedia Ionic Bond Metal To Nonmetal There are four kinds of bonding types to be aware of. How do ionic bonds form. Ionic bonding forms between two atoms when an electron is transferred from one. Electronegativity is a property of an atom, measuring how strongly it attracts or. Binary ionic compounds are composed of just two elements: By definition, a metal is relatively stable if it. Ionic Bond Metal To Nonmetal.
From slideplayer.com
NonMetals Metalloids Metals Periods Families(Groups) ppt download Ionic Bond Metal To Nonmetal Binary ionic compounds are composed of just two elements: How do ionic bonds form. By definition, a metal is relatively stable if it loses electrons to form a complete valence shell and becomes positively charged. Otherwise, consult a chart of electronegativity values. There are four kinds of bonding types to be aware of. Electronegativity is a property of an atom,. Ionic Bond Metal To Nonmetal.
From www.slideserve.com
PPT Atomic Systems and Bonding PowerPoint Presentation, free Ionic Bond Metal To Nonmetal Otherwise, consult a chart of electronegativity values. Electronegativity is a property of an atom, measuring how strongly it attracts or. A metal (which forms the cations) and a nonmetal (which forms the anions). A metal bonding to a nonmetal forms an ionic bond, while two nonmetals form a covalent bond. Ionic bonding forms between two atoms when an electron is. Ionic Bond Metal To Nonmetal.
From slidetodoc.com
Ionic Bonding Ionic Bonding What Is It Ionic Ionic Bond Metal To Nonmetal There are four kinds of bonding types to be aware of. A metal bonding to a nonmetal forms an ionic bond, while two nonmetals form a covalent bond. Otherwise, consult a chart of electronegativity values. Binary ionic compounds are composed of just two elements: Electronegativity is a property of an atom, measuring how strongly it attracts or. By definition, a. Ionic Bond Metal To Nonmetal.
From slideplayer.com
Chemical Bonds Notes 10/16/ ppt download Ionic Bond Metal To Nonmetal Ionic bonding forms between two atoms when an electron is transferred from one. A metal (which forms the cations) and a nonmetal (which forms the anions). Otherwise, consult a chart of electronegativity values. A metal bonding to a nonmetal forms an ionic bond, while two nonmetals form a covalent bond. There are four kinds of bonding types to be aware. Ionic Bond Metal To Nonmetal.
From www.youtube.com
Ionic bonds Reaction of metals & Nonmetals Metals and non metals Ionic Bond Metal To Nonmetal Ionic bonding forms between two atoms when an electron is transferred from one. A metal (which forms the cations) and a nonmetal (which forms the anions). Binary ionic compounds are composed of just two elements: By definition, a metal is relatively stable if it loses electrons to form a complete valence shell and becomes positively charged. A metal bonding to. Ionic Bond Metal To Nonmetal.
From slidetodoc.com
FLASHBACK How many electrons do all atoms want Ionic Bond Metal To Nonmetal Ionic bonding forms between two atoms when an electron is transferred from one. There are four kinds of bonding types to be aware of. Binary ionic compounds are composed of just two elements: By definition, a metal is relatively stable if it loses electrons to form a complete valence shell and becomes positively charged. Otherwise, consult a chart of electronegativity. Ionic Bond Metal To Nonmetal.
From www.chegg.com
Solved When metals bond with nonmetals, electrons are Ionic Bond Metal To Nonmetal Otherwise, consult a chart of electronegativity values. Ionic bonding forms between two atoms when an electron is transferred from one. A metal bonding to a nonmetal forms an ionic bond, while two nonmetals form a covalent bond. A metal (which forms the cations) and a nonmetal (which forms the anions). Electronegativity is a property of an atom, measuring how strongly. Ionic Bond Metal To Nonmetal.
From www.slideserve.com
PPT Chemistry IANB Page 11 PowerPoint Presentation, free download Ionic Bond Metal To Nonmetal A metal bonding to a nonmetal forms an ionic bond, while two nonmetals form a covalent bond. A metal (which forms the cations) and a nonmetal (which forms the anions). There are four kinds of bonding types to be aware of. How do ionic bonds form. Otherwise, consult a chart of electronegativity values. Electronegativity is a property of an atom,. Ionic Bond Metal To Nonmetal.
From slideplayer.com
Chemical Bonding. ppt download Ionic Bond Metal To Nonmetal A metal bonding to a nonmetal forms an ionic bond, while two nonmetals form a covalent bond. How do ionic bonds form. Otherwise, consult a chart of electronegativity values. Binary ionic compounds are composed of just two elements: A metal (which forms the cations) and a nonmetal (which forms the anions). There are four kinds of bonding types to be. Ionic Bond Metal To Nonmetal.
From slideplayer.com
Chemical Bonding A chemical bond is the attraction between atoms that Ionic Bond Metal To Nonmetal A metal (which forms the cations) and a nonmetal (which forms the anions). Otherwise, consult a chart of electronegativity values. Electronegativity is a property of an atom, measuring how strongly it attracts or. How do ionic bonds form. Ionic bonding forms between two atoms when an electron is transferred from one. By definition, a metal is relatively stable if it. Ionic Bond Metal To Nonmetal.
From slidetodoc.com
Unit 12 Chemical Bonding Molecular Geometry Definitions Chemical Ionic Bond Metal To Nonmetal Ionic bonding forms between two atoms when an electron is transferred from one. How do ionic bonds form. Binary ionic compounds are composed of just two elements: By definition, a metal is relatively stable if it loses electrons to form a complete valence shell and becomes positively charged. Electronegativity is a property of an atom, measuring how strongly it attracts. Ionic Bond Metal To Nonmetal.
From www.chegg.com
Solved When metals bond with nonmetals, electrons are Ionic Bond Metal To Nonmetal Binary ionic compounds are composed of just two elements: By definition, a metal is relatively stable if it loses electrons to form a complete valence shell and becomes positively charged. A metal (which forms the cations) and a nonmetal (which forms the anions). Otherwise, consult a chart of electronegativity values. How do ionic bonds form. A metal bonding to a. Ionic Bond Metal To Nonmetal.
From slidetodoc.com
Chemistry Ions Ions Ionic bonding occurs between metals Ionic Bond Metal To Nonmetal How do ionic bonds form. Otherwise, consult a chart of electronegativity values. By definition, a metal is relatively stable if it loses electrons to form a complete valence shell and becomes positively charged. A metal (which forms the cations) and a nonmetal (which forms the anions). Ionic bonding forms between two atoms when an electron is transferred from one. Binary. Ionic Bond Metal To Nonmetal.
From www.slideserve.com
PPT Chapter 1 Chemical Bonding PowerPoint Presentation, free download Ionic Bond Metal To Nonmetal How do ionic bonds form. A metal (which forms the cations) and a nonmetal (which forms the anions). Ionic bonding forms between two atoms when an electron is transferred from one. Binary ionic compounds are composed of just two elements: Electronegativity is a property of an atom, measuring how strongly it attracts or. There are four kinds of bonding types. Ionic Bond Metal To Nonmetal.
From stock.adobe.com
Ionic bond and electrostatic attraction from chemical bonding outline Ionic Bond Metal To Nonmetal Ionic bonding forms between two atoms when an electron is transferred from one. There are four kinds of bonding types to be aware of. Electronegativity is a property of an atom, measuring how strongly it attracts or. Binary ionic compounds are composed of just two elements: Otherwise, consult a chart of electronegativity values. A metal bonding to a nonmetal forms. Ionic Bond Metal To Nonmetal.
From slideplayer.com
Ionic Bonding Ken Rogers Miami Killian. ppt download Ionic Bond Metal To Nonmetal There are four kinds of bonding types to be aware of. Electronegativity is a property of an atom, measuring how strongly it attracts or. A metal (which forms the cations) and a nonmetal (which forms the anions). How do ionic bonds form. Otherwise, consult a chart of electronegativity values. Binary ionic compounds are composed of just two elements: By definition,. Ionic Bond Metal To Nonmetal.
From www.slideserve.com
PPT Bonding Between Atoms PowerPoint Presentation, free download ID Ionic Bond Metal To Nonmetal By definition, a metal is relatively stable if it loses electrons to form a complete valence shell and becomes positively charged. A metal (which forms the cations) and a nonmetal (which forms the anions). Electronegativity is a property of an atom, measuring how strongly it attracts or. A metal bonding to a nonmetal forms an ionic bond, while two nonmetals. Ionic Bond Metal To Nonmetal.
From www.jagranjosh.com
What are Ionic Compounds and how they are formed? Ionic Bond Metal To Nonmetal A metal (which forms the cations) and a nonmetal (which forms the anions). There are four kinds of bonding types to be aware of. Ionic bonding forms between two atoms when an electron is transferred from one. Otherwise, consult a chart of electronegativity values. A metal bonding to a nonmetal forms an ionic bond, while two nonmetals form a covalent. Ionic Bond Metal To Nonmetal.
From www.slideserve.com
PPT CHAPTER 2 A tomic structure and interatomic bonding PowerPoint Ionic Bond Metal To Nonmetal Ionic bonding forms between two atoms when an electron is transferred from one. A metal (which forms the cations) and a nonmetal (which forms the anions). By definition, a metal is relatively stable if it loses electrons to form a complete valence shell and becomes positively charged. Binary ionic compounds are composed of just two elements: Electronegativity is a property. Ionic Bond Metal To Nonmetal.