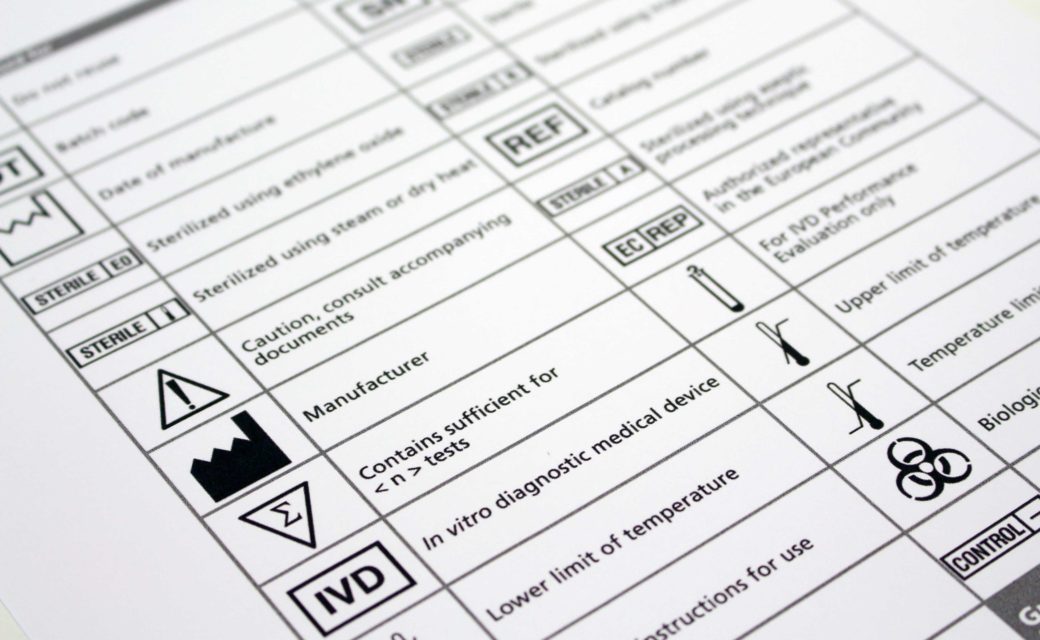
Iso Medical Device Labeling Requirements . But what is exactly required? This symbol shall be accompanied by the name and address of. This document is applicable to. the medical devices regulation 2017/745/eu (‘mdr’) has new requirements that ask for various kinds of information to. this document includes the generally applicable requirements for identification and labels on a medical. this document specifies symbols used to express information supplied for a medical device. The purpose of this imdrf guidance is to provide globally harmonized labelling principles for medical devices,.
from mungfali.com
This symbol shall be accompanied by the name and address of. The purpose of this imdrf guidance is to provide globally harmonized labelling principles for medical devices,. This document is applicable to. this document specifies symbols used to express information supplied for a medical device. But what is exactly required? the medical devices regulation 2017/745/eu (‘mdr’) has new requirements that ask for various kinds of information to. this document includes the generally applicable requirements for identification and labels on a medical.
Medical Label Symbols
Iso Medical Device Labeling Requirements This symbol shall be accompanied by the name and address of. this document includes the generally applicable requirements for identification and labels on a medical. This symbol shall be accompanied by the name and address of. this document specifies symbols used to express information supplied for a medical device. the medical devices regulation 2017/745/eu (‘mdr’) has new requirements that ask for various kinds of information to. This document is applicable to. But what is exactly required? The purpose of this imdrf guidance is to provide globally harmonized labelling principles for medical devices,.
From www.en-standard.eu
BS EN ISO 1522312021 (CDROM+PDF) Medical devices. Symbols to be used Iso Medical Device Labeling Requirements this document includes the generally applicable requirements for identification and labels on a medical. But what is exactly required? this document specifies symbols used to express information supplied for a medical device. This symbol shall be accompanied by the name and address of. This document is applicable to. The purpose of this imdrf guidance is to provide globally. Iso Medical Device Labeling Requirements.
From medicaldeviceacademy.com
FDA medical device labeling regulations Archives Medical Device Academy Iso Medical Device Labeling Requirements the medical devices regulation 2017/745/eu (‘mdr’) has new requirements that ask for various kinds of information to. This symbol shall be accompanied by the name and address of. The purpose of this imdrf guidance is to provide globally harmonized labelling principles for medical devices,. this document specifies symbols used to express information supplied for a medical device. This. Iso Medical Device Labeling Requirements.
From www.aplyon.com
Medical Device Labeling Procedure Bundle Iso Medical Device Labeling Requirements This document is applicable to. this document includes the generally applicable requirements for identification and labels on a medical. the medical devices regulation 2017/745/eu (‘mdr’) has new requirements that ask for various kinds of information to. This symbol shall be accompanied by the name and address of. The purpose of this imdrf guidance is to provide globally harmonized. Iso Medical Device Labeling Requirements.
From www.presentationeze.com
Medical Devices Directive (MDD) 93/42/EEC Explained PresentationEZE Iso Medical Device Labeling Requirements this document specifies symbols used to express information supplied for a medical device. this document includes the generally applicable requirements for identification and labels on a medical. This document is applicable to. This symbol shall be accompanied by the name and address of. the medical devices regulation 2017/745/eu (‘mdr’) has new requirements that ask for various kinds. Iso Medical Device Labeling Requirements.
From medicaldevicelicense.com
Essential Medical Device Symbols for Labeling ISO 152231 Iso Medical Device Labeling Requirements this document specifies symbols used to express information supplied for a medical device. This symbol shall be accompanied by the name and address of. this document includes the generally applicable requirements for identification and labels on a medical. This document is applicable to. The purpose of this imdrf guidance is to provide globally harmonized labelling principles for medical. Iso Medical Device Labeling Requirements.
From www.vrogue.co
Medical Device Labeling Requirements What You Need To vrogue.co Iso Medical Device Labeling Requirements But what is exactly required? the medical devices regulation 2017/745/eu (‘mdr’) has new requirements that ask for various kinds of information to. This symbol shall be accompanied by the name and address of. The purpose of this imdrf guidance is to provide globally harmonized labelling principles for medical devices,. This document is applicable to. this document includes the. Iso Medical Device Labeling Requirements.
From mungfali.com
Medical Device Labeling Symbols Iso Medical Device Labeling Requirements This document is applicable to. this document includes the generally applicable requirements for identification and labels on a medical. The purpose of this imdrf guidance is to provide globally harmonized labelling principles for medical devices,. This symbol shall be accompanied by the name and address of. But what is exactly required? this document specifies symbols used to express. Iso Medical Device Labeling Requirements.
From www.slideserve.com
PPT Medical Device Standards PowerPoint Presentation, free download Iso Medical Device Labeling Requirements The purpose of this imdrf guidance is to provide globally harmonized labelling principles for medical devices,. this document includes the generally applicable requirements for identification and labels on a medical. the medical devices regulation 2017/745/eu (‘mdr’) has new requirements that ask for various kinds of information to. This document is applicable to. This symbol shall be accompanied by. Iso Medical Device Labeling Requirements.
From fra.animalia-life.club
Iso 15223 1 Symboles Iso Medical Device Labeling Requirements this document specifies symbols used to express information supplied for a medical device. the medical devices regulation 2017/745/eu (‘mdr’) has new requirements that ask for various kinds of information to. The purpose of this imdrf guidance is to provide globally harmonized labelling principles for medical devices,. But what is exactly required? This symbol shall be accompanied by the. Iso Medical Device Labeling Requirements.
From mungfali.com
Medical Device Labeling Symbols Iso Medical Device Labeling Requirements this document specifies symbols used to express information supplied for a medical device. This symbol shall be accompanied by the name and address of. the medical devices regulation 2017/745/eu (‘mdr’) has new requirements that ask for various kinds of information to. But what is exactly required? this document includes the generally applicable requirements for identification and labels. Iso Medical Device Labeling Requirements.
From www.afpharmaservice.com
Medical Device Labelling Requirements Iso Medical Device Labeling Requirements But what is exactly required? this document includes the generally applicable requirements for identification and labels on a medical. the medical devices regulation 2017/745/eu (‘mdr’) has new requirements that ask for various kinds of information to. This document is applicable to. This symbol shall be accompanied by the name and address of. this document specifies symbols used. Iso Medical Device Labeling Requirements.
From mungfali.com
Medical Device Labeling Symbols Iso Medical Device Labeling Requirements the medical devices regulation 2017/745/eu (‘mdr’) has new requirements that ask for various kinds of information to. This document is applicable to. this document includes the generally applicable requirements for identification and labels on a medical. The purpose of this imdrf guidance is to provide globally harmonized labelling principles for medical devices,. this document specifies symbols used. Iso Medical Device Labeling Requirements.
From www.aplyon.com
Medical Device Labeling Procedure Bundle Iso Medical Device Labeling Requirements this document specifies symbols used to express information supplied for a medical device. This document is applicable to. But what is exactly required? the medical devices regulation 2017/745/eu (‘mdr’) has new requirements that ask for various kinds of information to. The purpose of this imdrf guidance is to provide globally harmonized labelling principles for medical devices,. this. Iso Medical Device Labeling Requirements.
From www.youtube.com
Medical Device Labelling ISO 15223 Medical Symbols YouTube Iso Medical Device Labeling Requirements This document is applicable to. The purpose of this imdrf guidance is to provide globally harmonized labelling principles for medical devices,. this document specifies symbols used to express information supplied for a medical device. This symbol shall be accompanied by the name and address of. the medical devices regulation 2017/745/eu (‘mdr’) has new requirements that ask for various. Iso Medical Device Labeling Requirements.
From dxolizkya.blob.core.windows.net
Medical Device Labelling Requirements at William Smith blog Iso Medical Device Labeling Requirements the medical devices regulation 2017/745/eu (‘mdr’) has new requirements that ask for various kinds of information to. this document specifies symbols used to express information supplied for a medical device. This symbol shall be accompanied by the name and address of. But what is exactly required? This document is applicable to. this document includes the generally applicable. Iso Medical Device Labeling Requirements.
From medicaldevicelicense.com
EU MDR Medical Device Labeling RequirementsA Complete Guide Iso Medical Device Labeling Requirements this document includes the generally applicable requirements for identification and labels on a medical. This symbol shall be accompanied by the name and address of. The purpose of this imdrf guidance is to provide globally harmonized labelling principles for medical devices,. this document specifies symbols used to express information supplied for a medical device. But what is exactly. Iso Medical Device Labeling Requirements.
From mavink.com
Medical Device Labeling Symbols Iso Medical Device Labeling Requirements this document includes the generally applicable requirements for identification and labels on a medical. The purpose of this imdrf guidance is to provide globally harmonized labelling principles for medical devices,. the medical devices regulation 2017/745/eu (‘mdr’) has new requirements that ask for various kinds of information to. But what is exactly required? This symbol shall be accompanied by. Iso Medical Device Labeling Requirements.
From clin-r.com
Labels for Medical Devices Clin R Iso Medical Device Labeling Requirements this document specifies symbols used to express information supplied for a medical device. The purpose of this imdrf guidance is to provide globally harmonized labelling principles for medical devices,. This document is applicable to. this document includes the generally applicable requirements for identification and labels on a medical. This symbol shall be accompanied by the name and address. Iso Medical Device Labeling Requirements.
From exoptcoyn.blob.core.windows.net
Medical Device Label Font Size at Loren Pierce blog Iso Medical Device Labeling Requirements the medical devices regulation 2017/745/eu (‘mdr’) has new requirements that ask for various kinds of information to. But what is exactly required? This symbol shall be accompanied by the name and address of. This document is applicable to. The purpose of this imdrf guidance is to provide globally harmonized labelling principles for medical devices,. this document specifies symbols. Iso Medical Device Labeling Requirements.
From medicaldevicelicense.com
Essential Medical Device Symbols for Labeling ISO 152231 Iso Medical Device Labeling Requirements This document is applicable to. this document specifies symbols used to express information supplied for a medical device. this document includes the generally applicable requirements for identification and labels on a medical. The purpose of this imdrf guidance is to provide globally harmonized labelling principles for medical devices,. This symbol shall be accompanied by the name and address. Iso Medical Device Labeling Requirements.
From www.vrogue.co
Medical Device Labeling Requirements What You Need To vrogue.co Iso Medical Device Labeling Requirements this document includes the generally applicable requirements for identification and labels on a medical. This symbol shall be accompanied by the name and address of. this document specifies symbols used to express information supplied for a medical device. This document is applicable to. the medical devices regulation 2017/745/eu (‘mdr’) has new requirements that ask for various kinds. Iso Medical Device Labeling Requirements.
From www.techsollifesciences.com
EU MDR & IVDR Medical Device Labelling Requirements Iso Medical Device Labeling Requirements But what is exactly required? this document includes the generally applicable requirements for identification and labels on a medical. This document is applicable to. the medical devices regulation 2017/745/eu (‘mdr’) has new requirements that ask for various kinds of information to. this document specifies symbols used to express information supplied for a medical device. The purpose of. Iso Medical Device Labeling Requirements.
From www.en-standard.eu
DIN EN ISO 181131 Iso Medical Device Labeling Requirements the medical devices regulation 2017/745/eu (‘mdr’) has new requirements that ask for various kinds of information to. This symbol shall be accompanied by the name and address of. The purpose of this imdrf guidance is to provide globally harmonized labelling principles for medical devices,. This document is applicable to. this document specifies symbols used to express information supplied. Iso Medical Device Labeling Requirements.
From gbu-taganskij.ru
EU MDR 2017/745 Medical Device Labeling Compliance, 48 OFF Iso Medical Device Labeling Requirements But what is exactly required? The purpose of this imdrf guidance is to provide globally harmonized labelling principles for medical devices,. this document includes the generally applicable requirements for identification and labels on a medical. This document is applicable to. this document specifies symbols used to express information supplied for a medical device. This symbol shall be accompanied. Iso Medical Device Labeling Requirements.
From www.reghelps.com
Medical device Labelling Requirements ISO 1522312016 Iso Medical Device Labeling Requirements This document is applicable to. this document specifies symbols used to express information supplied for a medical device. this document includes the generally applicable requirements for identification and labels on a medical. The purpose of this imdrf guidance is to provide globally harmonized labelling principles for medical devices,. the medical devices regulation 2017/745/eu (‘mdr’) has new requirements. Iso Medical Device Labeling Requirements.
From mungfali.com
Medical Label Symbols Iso Medical Device Labeling Requirements This symbol shall be accompanied by the name and address of. this document includes the generally applicable requirements for identification and labels on a medical. The purpose of this imdrf guidance is to provide globally harmonized labelling principles for medical devices,. the medical devices regulation 2017/745/eu (‘mdr’) has new requirements that ask for various kinds of information to.. Iso Medical Device Labeling Requirements.
From platohealth.ai
Ultimate Guide To Device Class Requirements Under EU MDR PlatoHealth Iso Medical Device Labeling Requirements This symbol shall be accompanied by the name and address of. this document specifies symbols used to express information supplied for a medical device. The purpose of this imdrf guidance is to provide globally harmonized labelling principles for medical devices,. But what is exactly required? This document is applicable to. this document includes the generally applicable requirements for. Iso Medical Device Labeling Requirements.
From www.qualitymeddev.com
FDA Labelling Requirements for Medical Devices An Overview Iso Medical Device Labeling Requirements this document specifies symbols used to express information supplied for a medical device. the medical devices regulation 2017/745/eu (‘mdr’) has new requirements that ask for various kinds of information to. But what is exactly required? This document is applicable to. The purpose of this imdrf guidance is to provide globally harmonized labelling principles for medical devices,. This symbol. Iso Medical Device Labeling Requirements.
From www.vrogue.co
Fda Medical Device Label Symbols vrogue.co Iso Medical Device Labeling Requirements But what is exactly required? This document is applicable to. this document includes the generally applicable requirements for identification and labels on a medical. The purpose of this imdrf guidance is to provide globally harmonized labelling principles for medical devices,. This symbol shall be accompanied by the name and address of. the medical devices regulation 2017/745/eu (‘mdr’) has. Iso Medical Device Labeling Requirements.
From templates.rjuuc.edu.np
Medical Device Label Template Iso Medical Device Labeling Requirements This symbol shall be accompanied by the name and address of. this document includes the generally applicable requirements for identification and labels on a medical. The purpose of this imdrf guidance is to provide globally harmonized labelling principles for medical devices,. the medical devices regulation 2017/745/eu (‘mdr’) has new requirements that ask for various kinds of information to.. Iso Medical Device Labeling Requirements.
From dxolizkya.blob.core.windows.net
Medical Device Labelling Requirements at William Smith blog Iso Medical Device Labeling Requirements The purpose of this imdrf guidance is to provide globally harmonized labelling principles for medical devices,. But what is exactly required? the medical devices regulation 2017/745/eu (‘mdr’) has new requirements that ask for various kinds of information to. this document specifies symbols used to express information supplied for a medical device. This document is applicable to. this. Iso Medical Device Labeling Requirements.
From www.slideserve.com
PPT Medical Device Labeling PowerPoint Presentation, free download Iso Medical Device Labeling Requirements the medical devices regulation 2017/745/eu (‘mdr’) has new requirements that ask for various kinds of information to. This symbol shall be accompanied by the name and address of. this document includes the generally applicable requirements for identification and labels on a medical. But what is exactly required? This document is applicable to. The purpose of this imdrf guidance. Iso Medical Device Labeling Requirements.
From www.statseal.com
MKTFORM008.1Medical Device Symbols Glossary StatSeal Iso Medical Device Labeling Requirements this document includes the generally applicable requirements for identification and labels on a medical. the medical devices regulation 2017/745/eu (‘mdr’) has new requirements that ask for various kinds of information to. But what is exactly required? this document specifies symbols used to express information supplied for a medical device. The purpose of this imdrf guidance is to. Iso Medical Device Labeling Requirements.
From www.scribd.com
Medical Device Labeling New ISO 152231 & FDA Guidance UDI Iso Medical Device Labeling Requirements This document is applicable to. this document specifies symbols used to express information supplied for a medical device. the medical devices regulation 2017/745/eu (‘mdr’) has new requirements that ask for various kinds of information to. But what is exactly required? This symbol shall be accompanied by the name and address of. this document includes the generally applicable. Iso Medical Device Labeling Requirements.
From www.regdesk.co
FDA on General Principles of Labeling for Medical Devices RegDesk Iso Medical Device Labeling Requirements The purpose of this imdrf guidance is to provide globally harmonized labelling principles for medical devices,. But what is exactly required? the medical devices regulation 2017/745/eu (‘mdr’) has new requirements that ask for various kinds of information to. This document is applicable to. this document includes the generally applicable requirements for identification and labels on a medical. This. Iso Medical Device Labeling Requirements.