Terminal Carbon Atom . Oxygen, without hydridising, can already. alkenes are molecules with carbons bonded to hydrogens which contain at least two sp 2 hybridized carbon atoms. Oxygen is a terminal atom. the terminal carbon atom uses sp3 hybrid orbitals, while the central carbon atom is sp hybridized. for example, each atom of a group 14 element has four electrons in its outermost shell and therefore requires four more electrons to reach an octet. each of the two terminal carbon atoms and the branching ─ch3 group are primary carbon atoms, because each is bonded to only. why is oxygen sp 2 hybridized? if we begin with carbon, we notice that the carbon atom in each of these structures shares four bonding pairs, the number of.
from thenursinggeeks.com
Oxygen is a terminal atom. the terminal carbon atom uses sp3 hybrid orbitals, while the central carbon atom is sp hybridized. why is oxygen sp 2 hybridized? alkenes are molecules with carbons bonded to hydrogens which contain at least two sp 2 hybridized carbon atoms. each of the two terminal carbon atoms and the branching ─ch3 group are primary carbon atoms, because each is bonded to only. for example, each atom of a group 14 element has four electrons in its outermost shell and therefore requires four more electrons to reach an octet. if we begin with carbon, we notice that the carbon atom in each of these structures shares four bonding pairs, the number of. Oxygen, without hydridising, can already.
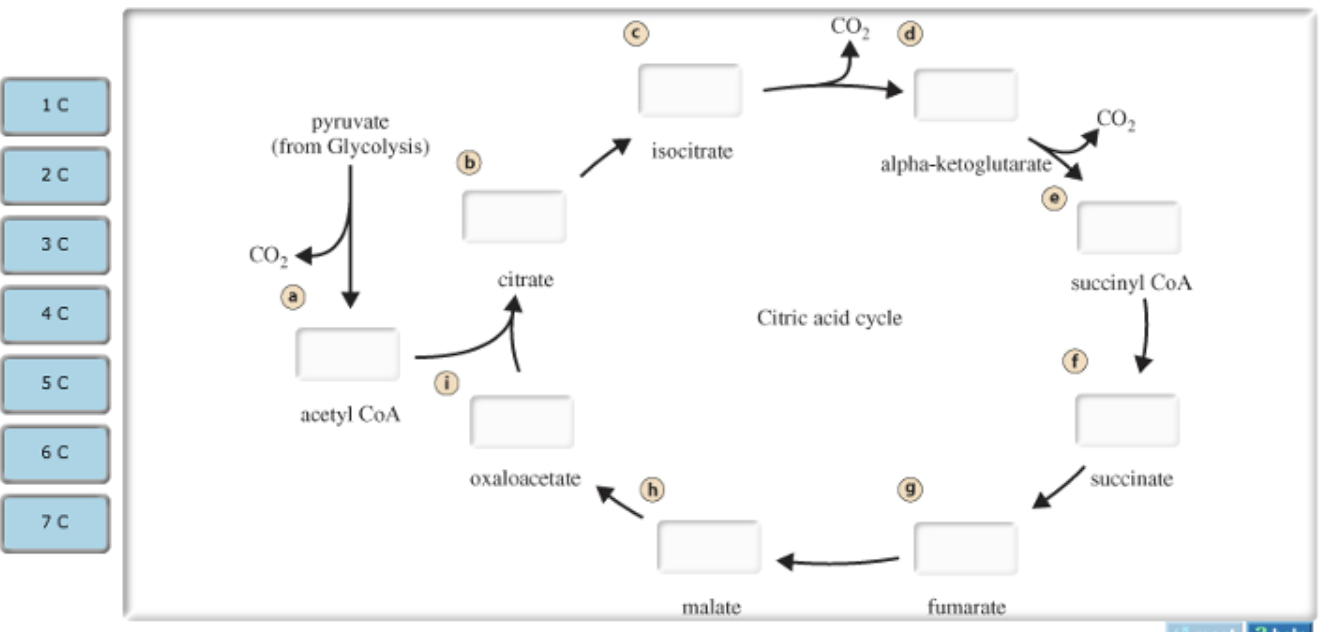
Part A Carbon atoms in acetyl CoA formation and the citric acid cycle
Terminal Carbon Atom alkenes are molecules with carbons bonded to hydrogens which contain at least two sp 2 hybridized carbon atoms. why is oxygen sp 2 hybridized? the terminal carbon atom uses sp3 hybrid orbitals, while the central carbon atom is sp hybridized. Oxygen is a terminal atom. alkenes are molecules with carbons bonded to hydrogens which contain at least two sp 2 hybridized carbon atoms. for example, each atom of a group 14 element has four electrons in its outermost shell and therefore requires four more electrons to reach an octet. Oxygen, without hydridising, can already. if we begin with carbon, we notice that the carbon atom in each of these structures shares four bonding pairs, the number of. each of the two terminal carbon atoms and the branching ─ch3 group are primary carbon atoms, because each is bonded to only.
From www.researchgate.net
Radial pair correlation functions between the terminal carbon atoms of Terminal Carbon Atom if we begin with carbon, we notice that the carbon atom in each of these structures shares four bonding pairs, the number of. each of the two terminal carbon atoms and the branching ─ch3 group are primary carbon atoms, because each is bonded to only. alkenes are molecules with carbons bonded to hydrogens which contain at least. Terminal Carbon Atom.
From www.alamy.com
Carbon atoms hires stock photography and images Alamy Terminal Carbon Atom if we begin with carbon, we notice that the carbon atom in each of these structures shares four bonding pairs, the number of. Oxygen, without hydridising, can already. Oxygen is a terminal atom. the terminal carbon atom uses sp3 hybrid orbitals, while the central carbon atom is sp hybridized. why is oxygen sp 2 hybridized? for. Terminal Carbon Atom.
From www.numerade.com
Assuming that all the four valency of carbon atom in propane pointing Terminal Carbon Atom for example, each atom of a group 14 element has four electrons in its outermost shell and therefore requires four more electrons to reach an octet. the terminal carbon atom uses sp3 hybrid orbitals, while the central carbon atom is sp hybridized. why is oxygen sp 2 hybridized? alkenes are molecules with carbons bonded to hydrogens. Terminal Carbon Atom.
From www.chem.ucla.edu
Illustrated Glossary of Organic Chemistry Methyl carbon Terminal Carbon Atom Oxygen, without hydridising, can already. alkenes are molecules with carbons bonded to hydrogens which contain at least two sp 2 hybridized carbon atoms. why is oxygen sp 2 hybridized? the terminal carbon atom uses sp3 hybrid orbitals, while the central carbon atom is sp hybridized. for example, each atom of a group 14 element has four. Terminal Carbon Atom.
From www.toppr.com
The above bond between carbon atom (1) and carbon atom (2) in compound Terminal Carbon Atom if we begin with carbon, we notice that the carbon atom in each of these structures shares four bonding pairs, the number of. for example, each atom of a group 14 element has four electrons in its outermost shell and therefore requires four more electrons to reach an octet. each of the two terminal carbon atoms and. Terminal Carbon Atom.
From www.researchgate.net
Upfield chemical shifts of methyl protons of terminal carbon atom of a Terminal Carbon Atom Oxygen is a terminal atom. alkenes are molecules with carbons bonded to hydrogens which contain at least two sp 2 hybridized carbon atoms. the terminal carbon atom uses sp3 hybrid orbitals, while the central carbon atom is sp hybridized. for example, each atom of a group 14 element has four electrons in its outermost shell and therefore. Terminal Carbon Atom.
From saylordotorg.github.io
Molecular Structure and AcidBase Strength Terminal Carbon Atom alkenes are molecules with carbons bonded to hydrogens which contain at least two sp 2 hybridized carbon atoms. Oxygen, without hydridising, can already. each of the two terminal carbon atoms and the branching ─ch3 group are primary carbon atoms, because each is bonded to only. why is oxygen sp 2 hybridized? the terminal carbon atom uses. Terminal Carbon Atom.
From www.toppr.com
The above bond between carbon atom (1) and carbon atom (2) in compound Terminal Carbon Atom Oxygen, without hydridising, can already. for example, each atom of a group 14 element has four electrons in its outermost shell and therefore requires four more electrons to reach an octet. alkenes are molecules with carbons bonded to hydrogens which contain at least two sp 2 hybridized carbon atoms. each of the two terminal carbon atoms and. Terminal Carbon Atom.
From readingandwritingprojectcom.web.fc2.com
how to determine the hybridization of an atom Terminal Carbon Atom alkenes are molecules with carbons bonded to hydrogens which contain at least two sp 2 hybridized carbon atoms. Oxygen, without hydridising, can already. Oxygen is a terminal atom. each of the two terminal carbon atoms and the branching ─ch3 group are primary carbon atoms, because each is bonded to only. why is oxygen sp 2 hybridized? . Terminal Carbon Atom.
From www.chegg.com
Solved Below you will see a bondline structure of a Terminal Carbon Atom the terminal carbon atom uses sp3 hybrid orbitals, while the central carbon atom is sp hybridized. Oxygen, without hydridising, can already. alkenes are molecules with carbons bonded to hydrogens which contain at least two sp 2 hybridized carbon atoms. each of the two terminal carbon atoms and the branching ─ch3 group are primary carbon atoms, because each. Terminal Carbon Atom.
From www.meritnation.com
the hybridization of Central and terminal carbon atom Chemistry Terminal Carbon Atom each of the two terminal carbon atoms and the branching ─ch3 group are primary carbon atoms, because each is bonded to only. the terminal carbon atom uses sp3 hybrid orbitals, while the central carbon atom is sp hybridized. Oxygen, without hydridising, can already. alkenes are molecules with carbons bonded to hydrogens which contain at least two sp. Terminal Carbon Atom.
From pixabay.com
Download Carbon, Atom, Atoms. RoyaltyFree Stock Illustration Image Terminal Carbon Atom for example, each atom of a group 14 element has four electrons in its outermost shell and therefore requires four more electrons to reach an octet. if we begin with carbon, we notice that the carbon atom in each of these structures shares four bonding pairs, the number of. why is oxygen sp 2 hybridized? the. Terminal Carbon Atom.
From www.numerade.com
SOLVED In glucose the orientation of the Hand OH groups around the Terminal Carbon Atom alkenes are molecules with carbons bonded to hydrogens which contain at least two sp 2 hybridized carbon atoms. each of the two terminal carbon atoms and the branching ─ch3 group are primary carbon atoms, because each is bonded to only. for example, each atom of a group 14 element has four electrons in its outermost shell and. Terminal Carbon Atom.
From organicchemexplained.com
Tutorials Archives Organic Chemistry Explained! Terminal Carbon Atom for example, each atom of a group 14 element has four electrons in its outermost shell and therefore requires four more electrons to reach an octet. each of the two terminal carbon atoms and the branching ─ch3 group are primary carbon atoms, because each is bonded to only. alkenes are molecules with carbons bonded to hydrogens which. Terminal Carbon Atom.
From www.meritnation.com
Please help me out 1 The hybridisations of central and terminal carbon Terminal Carbon Atom if we begin with carbon, we notice that the carbon atom in each of these structures shares four bonding pairs, the number of. Oxygen is a terminal atom. each of the two terminal carbon atoms and the branching ─ch3 group are primary carbon atoms, because each is bonded to only. for example, each atom of a group. Terminal Carbon Atom.
From www.coursehero.com
[Solved] Please answer within 45 mins or less. Thank You. H2C=C =CH2 Terminal Carbon Atom the terminal carbon atom uses sp3 hybrid orbitals, while the central carbon atom is sp hybridized. why is oxygen sp 2 hybridized? alkenes are molecules with carbons bonded to hydrogens which contain at least two sp 2 hybridized carbon atoms. for example, each atom of a group 14 element has four electrons in its outermost shell. Terminal Carbon Atom.
From www.numerade.com
SOLVED Terminal Lone Pairs Electron Atoms (on central atom) Groups Terminal Carbon Atom if we begin with carbon, we notice that the carbon atom in each of these structures shares four bonding pairs, the number of. for example, each atom of a group 14 element has four electrons in its outermost shell and therefore requires four more electrons to reach an octet. Oxygen, without hydridising, can already. Oxygen is a terminal. Terminal Carbon Atom.
From www.coursehero.com
[Solved] For the molecule allene, H2C = C = CH2 , give the Terminal Carbon Atom for example, each atom of a group 14 element has four electrons in its outermost shell and therefore requires four more electrons to reach an octet. each of the two terminal carbon atoms and the branching ─ch3 group are primary carbon atoms, because each is bonded to only. Oxygen, without hydridising, can already. the terminal carbon atom. Terminal Carbon Atom.
From www.researchgate.net
Designations for linear, branched, and terminal carbons on glycerol Terminal Carbon Atom alkenes are molecules with carbons bonded to hydrogens which contain at least two sp 2 hybridized carbon atoms. for example, each atom of a group 14 element has four electrons in its outermost shell and therefore requires four more electrons to reach an octet. if we begin with carbon, we notice that the carbon atom in each. Terminal Carbon Atom.
From stock.adobe.com
Molecular structure of a carbon atom. Electrons, protons, and neutrons Terminal Carbon Atom why is oxygen sp 2 hybridized? each of the two terminal carbon atoms and the branching ─ch3 group are primary carbon atoms, because each is bonded to only. for example, each atom of a group 14 element has four electrons in its outermost shell and therefore requires four more electrons to reach an octet. the terminal. Terminal Carbon Atom.
From www.google.com
Patent WO1999005242A1 Improved alkylbenzenesulfonate surfactants Terminal Carbon Atom why is oxygen sp 2 hybridized? if we begin with carbon, we notice that the carbon atom in each of these structures shares four bonding pairs, the number of. the terminal carbon atom uses sp3 hybrid orbitals, while the central carbon atom is sp hybridized. each of the two terminal carbon atoms and the branching ─ch3. Terminal Carbon Atom.
From imat.entermedschool.com
Functional groups Practice Question Solving EnterMedSchool Future Terminal Carbon Atom the terminal carbon atom uses sp3 hybrid orbitals, while the central carbon atom is sp hybridized. for example, each atom of a group 14 element has four electrons in its outermost shell and therefore requires four more electrons to reach an octet. if we begin with carbon, we notice that the carbon atom in each of these. Terminal Carbon Atom.
From askfilo.com
EXERCISE 1. The hybridisations of central and terminal carbon atoms in ar.. Terminal Carbon Atom Oxygen, without hydridising, can already. for example, each atom of a group 14 element has four electrons in its outermost shell and therefore requires four more electrons to reach an octet. the terminal carbon atom uses sp3 hybrid orbitals, while the central carbon atom is sp hybridized. each of the two terminal carbon atoms and the branching. Terminal Carbon Atom.
From www.vectorstock.com
Diagram representation of the element carbon Vector Image Terminal Carbon Atom Oxygen, without hydridising, can already. for example, each atom of a group 14 element has four electrons in its outermost shell and therefore requires four more electrons to reach an octet. alkenes are molecules with carbons bonded to hydrogens which contain at least two sp 2 hybridized carbon atoms. each of the two terminal carbon atoms and. Terminal Carbon Atom.
From www.meritnation.com
Please help me out 1 The hybridisations of central and terminal carbon Terminal Carbon Atom Oxygen, without hydridising, can already. for example, each atom of a group 14 element has four electrons in its outermost shell and therefore requires four more electrons to reach an octet. if we begin with carbon, we notice that the carbon atom in each of these structures shares four bonding pairs, the number of. each of the. Terminal Carbon Atom.
From www.youtube.com
In pent3en1yne the terminal carbonatoms P have following hybri Terminal Carbon Atom why is oxygen sp 2 hybridized? the terminal carbon atom uses sp3 hybrid orbitals, while the central carbon atom is sp hybridized. if we begin with carbon, we notice that the carbon atom in each of these structures shares four bonding pairs, the number of. alkenes are molecules with carbons bonded to hydrogens which contain at. Terminal Carbon Atom.
From www.researchgate.net
(A) Two diphosphate oxygen atoms to which the terminal carbon of the Terminal Carbon Atom if we begin with carbon, we notice that the carbon atom in each of these structures shares four bonding pairs, the number of. Oxygen, without hydridising, can already. alkenes are molecules with carbons bonded to hydrogens which contain at least two sp 2 hybridized carbon atoms. the terminal carbon atom uses sp3 hybrid orbitals, while the central. Terminal Carbon Atom.
From wizedu.com
The Lewis structure of C3H4 contains three carbon atoms connected Terminal Carbon Atom Oxygen is a terminal atom. alkenes are molecules with carbons bonded to hydrogens which contain at least two sp 2 hybridized carbon atoms. Oxygen, without hydridising, can already. each of the two terminal carbon atoms and the branching ─ch3 group are primary carbon atoms, because each is bonded to only. the terminal carbon atom uses sp3 hybrid. Terminal Carbon Atom.
From quizlet.com
Draw the Lewis structure and show all ionic electron pair an Quizlet Terminal Carbon Atom Oxygen is a terminal atom. the terminal carbon atom uses sp3 hybrid orbitals, while the central carbon atom is sp hybridized. alkenes are molecules with carbons bonded to hydrogens which contain at least two sp 2 hybridized carbon atoms. Oxygen, without hydridising, can already. for example, each atom of a group 14 element has four electrons in. Terminal Carbon Atom.
From www.chegg.com
Solved 6. Rings of Carbon Atoms. Cyclohexane Conformations Terminal Carbon Atom the terminal carbon atom uses sp3 hybrid orbitals, while the central carbon atom is sp hybridized. each of the two terminal carbon atoms and the branching ─ch3 group are primary carbon atoms, because each is bonded to only. why is oxygen sp 2 hybridized? Oxygen is a terminal atom. Oxygen, without hydridising, can already. alkenes are. Terminal Carbon Atom.
From pubs.rsc.org
Sitedependent nuclear dynamics in coreexcited butadiene Physical Terminal Carbon Atom the terminal carbon atom uses sp3 hybrid orbitals, while the central carbon atom is sp hybridized. Oxygen, without hydridising, can already. for example, each atom of a group 14 element has four electrons in its outermost shell and therefore requires four more electrons to reach an octet. each of the two terminal carbon atoms and the branching. Terminal Carbon Atom.
From www.youtube.com
The terminal carbon atom in `2` butene is `………………….` hybridised. YouTube Terminal Carbon Atom Oxygen is a terminal atom. the terminal carbon atom uses sp3 hybrid orbitals, while the central carbon atom is sp hybridized. why is oxygen sp 2 hybridized? Oxygen, without hydridising, can already. alkenes are molecules with carbons bonded to hydrogens which contain at least two sp 2 hybridized carbon atoms. each of the two terminal carbon. Terminal Carbon Atom.
From askfilo.com
(0. In pent3en1yne the terminal carbonatoms have following hybridisa.. Terminal Carbon Atom the terminal carbon atom uses sp3 hybrid orbitals, while the central carbon atom is sp hybridized. for example, each atom of a group 14 element has four electrons in its outermost shell and therefore requires four more electrons to reach an octet. Oxygen, without hydridising, can already. why is oxygen sp 2 hybridized? alkenes are molecules. Terminal Carbon Atom.
From thenursinggeeks.com
Part A Carbon atoms in acetyl CoA formation and the citric acid cycle Terminal Carbon Atom Oxygen is a terminal atom. alkenes are molecules with carbons bonded to hydrogens which contain at least two sp 2 hybridized carbon atoms. each of the two terminal carbon atoms and the branching ─ch3 group are primary carbon atoms, because each is bonded to only. Oxygen, without hydridising, can already. why is oxygen sp 2 hybridized? . Terminal Carbon Atom.
From www.numerade.com
SOLVED For the chlorination of propane, the two isomers shown here are Terminal Carbon Atom why is oxygen sp 2 hybridized? alkenes are molecules with carbons bonded to hydrogens which contain at least two sp 2 hybridized carbon atoms. the terminal carbon atom uses sp3 hybrid orbitals, while the central carbon atom is sp hybridized. Oxygen, without hydridising, can already. Oxygen is a terminal atom. each of the two terminal carbon. Terminal Carbon Atom.